FDA records indicate that there are no current recalls for this drug.
Are you a medical professional?
Trending Topics
Sentroxatine Recall
Get an alert when a recall is issued.
Questions & Answers
Side Effects & Adverse Reactions
There is currently no warning information available for this product. We apologize for any inconvenience.
Legal Issues
There is currently no legal information available for this drug.
FDA Safety Alerts
There are currently no FDA safety alerts available for this drug.
Manufacturer Warnings
There is currently no manufacturer warning information available for this drug.
FDA Labeling Changes
There are currently no FDA labeling changes available for this drug.
Uses
Enter section text here
1 INDICATIONS AND USAGE
1.1 Major Depressive Disorder
Fluoxetine is indicated for the acute and maintenance treatment of Major Depressive Disorder in adult patients and in pediatric patients aged 8 to18 years [see Clinical Studies (14.1)].
The usefulness of the drug in adult and pediatric patients receiving fluoxetine for extended periods, should periodically be re-evaluated [see Dosage and Administration (2.1)].
1.2 Obsessive Compulsive Disorder
Fluoxetine is indicated for the acute and maintenance treatment of obsessions and compulsions in adult patients and in pediatric patients aged 7 to 17 years with Obsessive Compulsive Disorder (OCD) [see Clinical Studies (14.2)].
The effectiveness of fluoxetine in long-term use, i.e., for more than 13 weeks, has not been systematically evaluated in placebo-controlled trials. Therefore, the physician who elects to use fluoxetine for extended periods, should periodically re-evaluate the long-term usefulness of the drug for the individual patient [see Dosage and Administration (2.2)].
1.3 Bulimia Nervosa
Fluoxetine is indicated for the acute and maintenance treatment of binge-eating and vomiting behaviors in adult patients with moderate to severe Bulimia Nervosa [see Clinical Studies (14.3)].
The physician who elects to use fluoxetine for extended periods should periodically re-evaluate the long-term usefulness of the drug for the individual patient [see Dosage and Administration (2.3)].
1.4 Panic Disorder
Fluoxetine is indicated for the acute treatment of Panic Disorder, with or without agoraphobia, in adult patients [see Clinical Studies (14.4)].
The effectiveness of fluoxetine in long-term use, i.e., for more than 12 weeks, has not been established in placebo-controlled trials. Therefore, the physician who elects to use fluoxetine for extended periods, should periodically re-evaluate the long-term usefulness of the drug for the individual patient [see Dosage and Administration (2.4) ].
INDICATIONS FOR USE
Sentra PM is intended for the clinical dietary management of the metabolic processes associated with sleep disorders.
History
There is currently no drug history available for this drug.
Other Information
11 DESCRIPTION
Fluoxetine Hydrochloride is a psychotropic drug for oral administration. It is also marketed for the treatment of premenstrual dysphoric disorder (Sarafem®, fluoxetine hydrochloride). It is designated (±)-N-methyl-3-phenyl-3-[(α,α,α-trifluoro-p-tolyl)oxy]propylamine hydrochloride. Fluoxetine Hydrochloride has the following structural formula:

C17H18F3NO•HCl M.W. 345.79
Fluoxetine hydrochloride is a white to off-white crystalline solid with a solubility of 14 mg/mL in water.
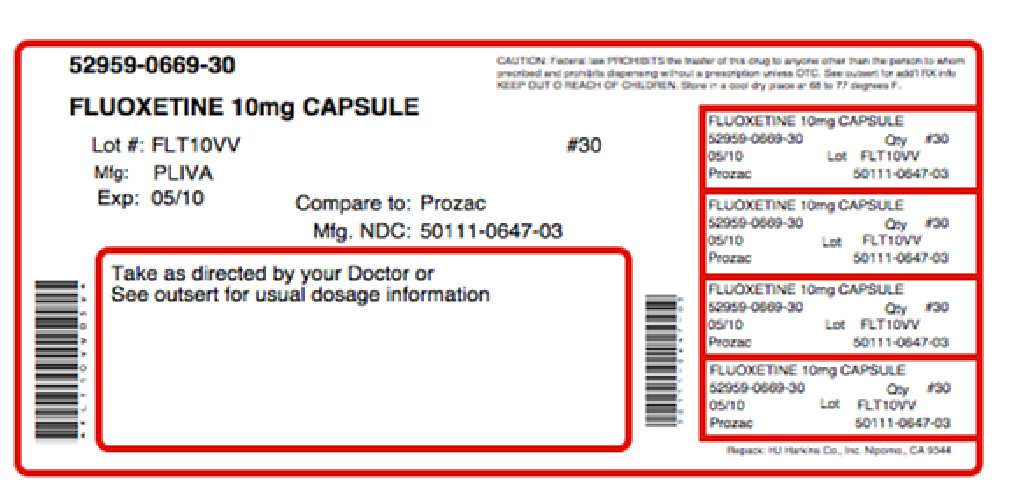
Each capsule, for oral administration, contains fluoxetine hydrochloride equivalent to 10 mg (32.3 μmol) or 20 mg (64.7 μmol) of fluoxetine. In addition, the capsules also contain the following inactive ingredients: ammonium hydroxide, DandC yellow #10, FDandC blue #1, gelatin, magnesium stearate, pregelatinized corn starch, propylene glycol, shellac, and titanium dioxide.
PRODUCT DESCRIPTION
Primary Ingredients
Sentra PM consists of a proprietary blend of amino acids, cocoa, ginkgo biloba and flavonoids in specific proportions. These ingredients fall into the category of “Generally Regarded as Safe” (GRAS) as defined by the Food and Drug Administration (FDA) (Sections 201(s) and 409 of the Federal Food, Drug, and Cosmetic Act). A GRAS substance is distinguished from a food additive on the basis of the common knowledge about the safety of the substance for its intended use. The standard for an ingredient to achieve GRAS status requires not only technical demonstration of non-toxicity and safety, but also general recognition of safety through widespread usage and agreement of that safety by experts in the field. Many ingredients have been determined by the U.S. Food and Drug Administration (FDA) to be GRAS, and are listed as such by regulation, in Volume 21 Code of Federal Regulations (CFR) Sections 182, 184, and 186.
Amino Acids
Amino Acids are the building blocks of protein. All amino acids are GRAS listed as they have been ingested by humans for thousands of years. The doses of the amino acids in Sentra PM are equivalent to those found in the usual human diet; however the formulation uses specific ratios of the key ingredients to elicit a therapeutic response. Patients with sleep disorders may require an increased amount of certain amino acids that cannot be obtained from normal diet alone. Tryptophan, for example, is an obligatory amino acid. The body cannot make tryptophan and must obtain tryptophan from the diet. Tryptophan is needed to produce serotonin. Serotonin is required to induce sleep. Patients with sleep disorders have altered serotonin metabolism. Some patients with sleep disorders have a resistance to the use of tryptophan that is similar to the mechanism found in insulin resistance that is genetically determined. Patients with sleep disorders frequently cannot acquire sufficient tryptophan from the diet without ingesting a prohibitively large amount of calories, particularly protein rich calories.
Flavonoids
Flavonoids are a group of phytochemical compounds found in all vascular plants including fruits and vegetables. They are a part of a larger class of compounds known as polyphenols. Many of the therapeutic or health benefits of colored fruits and vegetables, cocoa, red wine, and green tea are directly related to their flavonoid content. The specially formulated flavonoids found in Sentra PM cannot be obtained from conventional foods in the necessary proportions to elicit a therapeutic response.
Other Ingredients
Sentra PM contains the following inactive or other ingredients, as fillers, excipients, and colorings: magnesium stearate, microcrystalline cellulose, Maltodextrin NF, gelatin (as the capsule material).
Physical Description
Sentra PM is a yellow to light brown powder. Sentra PM contains L-Glutamic Acid, 5-Hydroxytryptophan as Griffonia Seed Extract, Acetylcarnitine HCL, Choline Bitartrate, Cinnamon, Cocoa, Ginkgo Biloba, and Hawthorn Berry.
Sources
Sentroxatine Manufacturers
-
Physician Therapeutics Llc
![Sentroxatine (Fluoxetine Hydrochloride, Choline) Kit [Physician Therapeutics Llc]](/wp-content/themes/bootstrap/assets/img/loading2.gif)
Sentroxatine | Physician Therapeutics Llc
![Sentroxatine (Fluoxetine Hydrochloride, Choline) Kit [Physician Therapeutics Llc] Sentroxatine (Fluoxetine Hydrochloride, Choline) Kit [Physician Therapeutics Llc]](/wp-content/themes/bootstrap/assets/img/loading2.gif)
Enter section text here
2 DOSAGE AND ADMINISTRATION
2.1 Major Depressive Disorder
Initial Treatment
Adult — In controlled trials used to support the efficacy of fluoxetine, patients were administered morning doses ranging from 20 to 80 mg/day. Studies comparing fluoxetine 20, 40, and 60 mg/day to placebo indicate that 20 mg/day is sufficient to obtain a satisfactory response in Major Depressive Disorder in most cases. Consequently, a dose of 20 mg/day, administered in the morning, is recommended as the initial dose.
A dose increase may be considered after several weeks if insufficient clinical improvement is observed. Doses above 20 mg/day may be administered on a once-a-day (morning) or BID schedule (i.e., morning and noon) and should not exceed a maximum dose of 80 mg/day.
Pediatric (children and adolescents) — In the short-term (8 to 9 week) controlled clinical trials of fluoxetine supporting its effectiveness in the treatment of Major Depressive Disorder, patients were administered fluoxetine doses of 10 to 20 mg/day [see Clinical Studies (14.1)]. Treatment should be initiated with a dose of 10 or 20 mg/day. After 1 week at 10 mg/day, the dose should be increased to 20 mg/day.
However, due to higher plasma levels in lower weight children, the starting and target dose in this group may be 10 mg/day. A dose increase to 20 mg/day may be considered after several weeks if insufficient clinical improvement is observed.
All patients — As with other drugs effective in the treatment of Major Depressive Disorder, the full effect may be delayed until 4 weeks of treatment or longer.
Maintenance/Continuation/Extended Treatment — It is generally agreed that acute episodes of Major Depressive Disorder require several months or longer of sustained pharmacologic therapy. Whether the dose needed to induce remission is identical to the dose needed to maintain and/or sustain euthymia is unknown.
Daily Dosing — Systematic evaluation of fluoxetine in adult patients has shown that its efficacy in Major Depressive Disorder is maintained for periods of up to 38 weeks following 12 weeks of open-label acute treatment (50 weeks total) at a dose of 20 mg/day [see Clinical Studies (14.1)].
Switching Patients to a Tricyclic Antidepressant (TCA) — Dosage of a TCA may need to be reduced, and plasma TCA concentrations may need to be monitored temporarily when fluoxetine is coadministered or has been recently discontinued [see Drug Interactions (7.9)].
Switching Patients to or From a Monoamine Oxidase Inhibitor (MAOI) — At least 14 days should elapse between discontinuation of an MAOI and initiation of therapy with fluoxetine. In addition, at least 5 weeks, perhaps longer, should be allowed after stopping fluoxetine before starting an MAOI [see Contraindications (4) and Drug Interactions (7.1)].
2.2 Obsessive-Compulsive Disorder
Initial Treatment
Adult — In the controlled clinical trials of fluoxetine supporting its effectiveness in the treatment of OCD, patients were administered fixed daily doses of 20, 40, or 60 mg of fluoxetine or placebo [see Clinical Studies (14.2)]. In one of these studies, no dose-response relationship for effectiveness was demonstrated. Consequently, a dose of 20 mg/day, administered in the morning, is recommended as the initial dose. Since there was a suggestion of a possible dose-response relationship for effectiveness in the second study, a dose increase may be considered after several weeks if insufficient clinical improvement is observed. The full therapeutic effect may be delayed until 5 weeks of treatment or longer.
Doses above 20 mg/day may be administered on a once daily (i.e., morning) or BID schedule (i.e., morning and noon). A dose range of 20 to 60 mg/day is recommended; however, doses of up to 80 mg/day have been well tolerated in open studies of OCD. The maximum fluoxetine dose should not exceed 80 mg/day.
Pediatric (children and adolescents) — In the controlled clinical trial of fluoxetine supporting its effectiveness in the treatment of OCD, patients were administered fluoxetine doses in the range of 10 to 60 mg/day [see Clinical Studies (14.2)].
In adolescents and higher weight children, treatment should be initiated with a dose of 10 mg/day. After 2 weeks, the dose should be increased to 20 mg/day. Additional dose increases may be considered after several more weeks if insufficient clinical improvement is observed. A dose range of 20 to 60 mg/day is recommended.
In lower weight children, treatment should be initiated with a dose of 10 mg/day. Additional dose increases may be considered after several more weeks if insufficient clinical improvement is observed. A dose range of 20 to 30 mg/day is recommended. Experience with daily doses greater than 20 mg is very minimal, and there is no experience with doses greater than 60 mg.
Maintenance/Continuation Treatment — While there are no systematic studies that answer the question of how long to continue fluoxetine, OCD is a chronic condition and it is reasonable to consider continuation for a responding patient. Although the efficacy of fluoxetine after 13 weeks has not been documented in controlled trials, adult patients have been continued in therapy under double-blind conditions for up to an additional 6 months without loss of benefit. However, dosage adjustments should be made to maintain the patient on the lowest effective dosage, and patients should be periodically reassessed to determine the need for treatment.
2.3 Bulimia Nervosa
Initial Treatment — In the controlled clinical trials of fluoxetine supporting its effectiveness in the treatment of Bulimia Nervosa, patients were administered fixed daily fluoxetine doses of 20 or 60 mg, or placebo [see Clinical Studies (14.3)]. Only the 60 mg dose was statistically significantly superior to placebo in reducing the frequency of binge-eating and vomiting. Consequently, the recommended dose is 60 mg/day, administered in the morning. For some patients it may be advisable to titrate up to this target dose over several days. Fluoxetine doses above 60 mg/day have not been systematically studied in patients with bulimia.
Maintenance/Continuation Treatment — Systematic evaluation of continuing fluoxetine 60 mg/day for periods of up to 52 weeks in patients with bulimia who have responded while taking fluoxetine 60 mg/day during an 8 week acute treatment phase has demonstrated a benefit of such maintenance treatment [see Clinical Studies (14.3)]. Nevertheless, patients should be periodically reassessed to determine the need for maintenance treatment.
2.4 Panic Disorder
Initial Treatment — In the controlled clinical trials of fluoxetine supporting its effectiveness in the treatment of Panic Disorder, patients were administered fluoxetine doses in the range of 10 to 60 mg/day [see Clinical Studies (14.4)]. Treatment should be initiated with a dose of 10 mg/day. After one week, the dose should be increased to 20 mg/day. The most frequently administered dose in the 2 flexible-dose clinical trials was 20 mg/day.
A dose increase may be considered after several weeks if no clinical improvement is observed. Fluoxetine doses above 60 mg/day have not been systematically evaluated in patients with Panic Disorder.
Maintenance/Continuation Treatment — While there are no systematic studies that answer the question of how long to continue fluoxetine, panic disorder is a chronic condition and it is reasonable to consider continuation for a responding patient. Nevertheless, patients should be periodically reassessed to determine the need for continued treatment.
2.7 Dosing in Specific Populations
Treatment of pregnant Women During the Third Trimester — When treating pregnant women with fluoxetine during the third trimester, the physician should carefully consider the potential risks and potential benefits of treatment. Neonates exposed to SNRIs or SSRIs late in the third trimester have developed complications requiring prolonged hospitalization, respiratory support, and tube feeding. The physician may consider tapering fluoxetine in the third trimester [see Use in Specific Populations (8.1)].
Geriatrics — A lower or less frequent dosage should be considered for the elderly [see Use in Specific Populations (8.5)]
Hepatic Impairment — As with many other medications, a lower or less frequent dosage should be used in patients with hepatic impairment [see Clinical Pharmacology (12.4) and Use in Specific Populations ( 8.6)].
Concomitant Illness — Patients with concurrent disease or on multiple concomitant medications may require dosage adjustments [see Clinical Pharmacology (12.4) and Warnings and Precautions (5.10)].
2.8 Discontinuation of Treatment
Symptoms associated with discontinuation of fluoxetine, SNRIs, and SSRIs, have been reported [see Warnings and Precautions (5.13)].DOSAGE AND ADMINISTRATION
Recommended Administration For the dietary management of the metabolic processes associated with sleep disorders. Take (2) capsules daily at bedtime. An additional dose of one or two capsules may be taken after awakenings during the night. As with most amino acid formulations Sentra PM should be taken without food to increase the absorption of key ingredients.
Login To Your Free Account