FDA records indicate that there are no current recalls for this drug.
Are you a medical professional?
Trending Topics
Tarceva Recall
Get an alert when a recall is issued.
Questions & Answers
Side Effects & Adverse Reactions
There is currently no warning information available for this product. We apologize for any inconvenience.
Legal Issues
There is currently no legal information available for this drug.
FDA Safety Alerts
There are currently no FDA safety alerts available for this drug.
Manufacturer Warnings
There is currently no manufacturer warning information available for this drug.
FDA Labeling Changes
There are currently no FDA labeling changes available for this drug.
Uses
Enter section text here
TARCEVA monotherapy is indicated for the treatment of patients with locally advanced or metastatic non-small cell lung cancer after failure of at least one prior chemotherapy regimen [see Clinical Studies (14.1)].
Results from two, multicenter, placebo-controlled, randomized, Phase 3 trials conducted in first-line patients with locally advanced or metastatic NSCLC showed no clinical benefit with the concurrent administration of TARCEVA with platinum-based chemotherapy [carboplatin and paclitaxel or gemcitabine and cisplatin] and its use is not recommended in that setting [see Clinical Studies (14.3)].
TARCEVA in combination with gemcitabine is indicated for the first-line treatment of patients with locally advanced, unresectable or metastatic pancreatic cancer [see Clinical Studies (14.3)].
History
There is currently no drug history available for this drug.
Other Information
TARCEVA (erlotinib), a kinase inhibitor, is a quinazolinamine with the chemical name N-(3-ethynylphenyl)-6,7-bis(2-methoxyethoxy)-4-quinazolinamine. TARCEVA contains erlotinib as the hydrochloride salt that has the following structural formula:
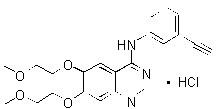
Erlotinib hydrochloride has the molecular formula C22H23N3O4.HCl and a molecular weight of 429.90. The molecule has a pKa of 5.42 at 25oC. Erlotinib hydrochloride is very slightly soluble in water, slightly soluble in methanol and practically insoluble in acetonitrile, acetone, ethyl acetate and hexane.
Aqueous solubility of erlotinib hydrochloride is dependent on pH with increased solubility at a pH of less than 5 due to protonation of the secondary amine. Over the pH range of 1.4 to 9.6, maximal solubility of approximately 0.4 mg/mL occurs at a pH of approximately 2.
TARCEVA tablets for oral administration are available in three dosage strengths containing erlotinib hydrochloride (27.3 mg, 109.3 mg and 163.9 mg) equivalent to 25 mg, 100 mg and 150 mg erlotinib and the following inactive ingredients: lactose monohydrate, hypromellose, hydroxypropyl cellulose, magnesium stearate, microcrystalline cellulose, sodium starch glycolate, sodium lauryl sulfate and titanium dioxide. The tablets also contain trace amounts of color additives, including FD&C Yellow #6 (25 mg only) for product identification.
Sources
Tarceva Manufacturers
-
Physicians Total Care, Inc.
![Tarceva (Erlotinib Hydrochloride) Tablet [Physicians Total Care, Inc.]](/wp-content/themes/bootstrap/assets/img/loading2.gif)
Tarceva | Physicians Total Care, Inc.
![Tarceva (Erlotinib Hydrochloride) Tablet [Physicians Total Care, Inc.] Tarceva (Erlotinib Hydrochloride) Tablet [Physicians Total Care, Inc.]](/wp-content/themes/bootstrap/assets/img/loading2.gif)
Enter section text here
2.1 Recommended Dose - NSCLCThe recommended daily dose of TARCEVA for non-small cell lung cancer is 150 mg taken at least one hour before or two hours after the ingestion of food. Treatment should continue until disease progression or unacceptable toxicity occurs. There is no evidence that treatment beyond progression is beneficial.
2.2 Recommended Dose - Pancreatic CancerThe recommended daily dose of TARCEVA for pancreatic cancer is 100 mg taken at least one hour before or two hours after the ingestion of food, in combination with gemcitabine (see the gemcitabine package insert). Treatment should continue until disease progression or unacceptable toxicity occurs.
2.3 Dose ModificationsIn patients who develop an acute onset of new or progressive pulmonary symptoms, such as dyspnea, cough or fever, treatment with TARCEVA should be interrupted pending diagnostic evaluation. If Interstitial Lung Disease (ILD) is diagnosed, TARCEVA should be discontinued and appropriate treatment instituted as necessary [see Warnings and Precautions (5.1)].
Diarrhea can usually be managed with loperamide. Patients with severe diarrhea who are unresponsive to loperamide or who become dehydrated may require dose reduction or temporary interruption of therapy. Patients with severe skin reactions may also require dose reduction or temporary interruption of therapy.
When dose reduction is necessary, the TARCEVA dose should be reduced in 50 mg decrements.
In patients who are taking TARCEVA with a strong CYP3A4 inhibitor such as, but not limited to, atazanavir, clarithromycin, indinavir, itraconazole, ketoconazole, nefazodone, nelfinavir, ritonavir, saquinavir, telithromycin, troleandomycin (TAO), voriconazole, or grapefruit or grapefruit juice, a dose reduction should be considered if severe adverse reactions occur. Similarly, in patients who are taking TARCEVA with an inhibitor of both CYP3A4 and CYP1A2 like ciprofloxacin, a dose reduction of TARCEVA should be considered if severe adverse reactions occur. [see Drug Interactions (7)].
Pre-treatment with the CYP3A4 inducer rifampicin decreased erlotinib AUC by about 2/3 to 4/5. Use of alternative treatments lacking CYP3A4 inducing activity is strongly recommended. If an alternative treatment is unavailable, an increase in the dose of TARCEVA should be considered as tolerated at two week intervals while monitoring the patient’s safety. The maximum dose of TARCEVA studied in combination with rifampicin is 450 mg. If the TARCEVA dose is adjusted upward, the dose will need to be reduced immediately to the indicated starting dose upon discontinuation of rifampicin or other inducers. Other CYP3A4 inducers include, but are not limited to rifabutin, rifapentine, phenytoin, carbamazepine, phenobarbital and St. John's Wort. These too should be avoided if possible [see Drug Interactions (7)].
Cigarette smoking has been shown to reduce erlotinib exposure. Patients should be advised to stop smoking. If a patient continues to smoke, a cautious increase in the dose of TARCEVA, not exceeding 300 mg may be considered, while monitoring the patient’s safety. However, efficacy and long-term safety (> 14 days) of a dose higher than the recommended starting doses have not been established in patients who continue to smoke cigarettes. If the TARCEVA dose is adjusted upward, the dose should be reduced immediately to the indicated starting dose upon cessation of smoking [see Clinical Pharmacology (12.3)].
Erlotinib is eliminated by hepatic metabolism and biliary excretion. Although erlotinib exposure was similar in patients with moderately impaired hepatic function (Child-Pugh B), patients with hepatic impairment (total bilirubin > ULN or Child-Pugh A, B and C) should be closely monitored during therapy with TARCEVA [see WARNINGS and PRECAUTIONS (5.2 )]. Treatment with TARCEVA should be used with extra caution in patients with total bilirubin > 3 x ULN. TARCEVA dosing should be interrupted or discontinued if changes in liver function are severe such as doubling of total bilirubin and/or tripling of transaminases in the setting of pretreatment values outside normal range. In the setting of worsening liver function tests, before they become severe, dose interruption and/or dose reduction with frequent liver function test monitoring should be considered. TARCEVA dosing should be interrupted or discontinued if total bilirubin is >3 x ULN and/or transaminases are >5 x ULN in the setting of normal pretreatment values [see Warnings and Precautions (5.2 , 5.3 ), Adverse Reactions (6.3) and Use in Specific Populations (8.6 )].
-
Genentech, Inc.
![Tarceva (Erlotinib Hydrochloride) Tablet [Genentech, Inc.]](/wp-content/themes/bootstrap/assets/img/loading2.gif)
Tarceva | Genentech, Inc.
![Tarceva (Erlotinib Hydrochloride) Tablet [Genentech, Inc.] Tarceva (Erlotinib Hydrochloride) Tablet [Genentech, Inc.]](/wp-content/themes/bootstrap/assets/img/loading2.gif)
2.1 Patient SelectionSelect patients for the first-line treatment of metastatic NSCLC with TARCEVA based on the presence of EGFR exon 19 deletions or exon 21 (L858R) substitution mutations in tumor specimens [See Clinical Studies (14.1)]. Information on FDA-approved tests for the detection of EGFR mutations in NSCLC is available at: http://www.fda.gov/CompanionDiagnostics
2.2 Recommended Dose – NSCLCThe recommended daily dose of TARCEVA for NSCLC is 150 mg taken on an empty stomach, i.e., at least one hour before or two hours after the ingestion of food. Treatment should continue until disease progression or unacceptable toxicity occurs.
2.3 Recommended Dose – Pancreatic CancerThe recommended daily dose of TARCEVA for pancreatic cancer is 100 mg taken once daily in combination with gemcitabine. Take TARCEVA on an empty stomach, i.e., at least one hour before or two hours after the ingestion of food. Treatment should continue until disease progression or unacceptable toxicity occurs [see Clinical Studies (14.5)].
2.4 Dose ModificationsDiscontinue TARCEVA for:
• Interstitial Lung Disease (ILD) [see Warnings and Precautions (5.1)]. • Severe hepatic toxicity that does not improve significantly or resolve within three weeks [see Warnings and Precautions (5.3)]. • Gastrointestinal perforation [see Warnings and Precautions (5.4)]. • Severe bullous, blistering or exfoliating skin conditions [see Warnings and Precautions (5.5)]. • Corneal perforation or severe ulceration [see Warnings and Precautions (5.9)].Withhold TARCEVA:
• During diagnostic evaluation for possible ILD. • For severe (CTCAE grade 3 to 4) renal toxicity, and consider discontinuation of TARCEVA [see Warnings and Precautions (5.2)]. • In patients without pre-existing hepatic impairment for total bilirubin levels greater than 3 times the upper limit of normal or transaminases greater than 5 times the upper limit of normal, and consider discontinuation of TARCEVA [see Warnings and Precautions (5.3)]. • In patients with pre-existing hepatic impairment or biliary obstruction for doubling of bilirubin or tripling of transaminases values over baseline and consider discontinuation of TARCEVA [see Warnings and Precautions (5.3)]. • For persistent severe diarrhea not responsive to medical management (e.g., loperamide). • For severe rash not responsive to medical management. • For keratitis of (NCI-CTC version 4.0) grade 3-4 or for grade 2 lasting more than 2 weeks [see Warnings and Precautions (5.9)]. • For acute/worsening ocular disorders such as eye pain, and consider discontinuation of TARCEVA [see Warnings and Precautions (5.9)].Reduce TARCEVA by 50 mg decrements:
• If severe reactions occur with concomitant use of strong CYP3A4 inhibitors [such as atazanavir, clarithromycin, indinavir, itraconazole, ketoconazole, nefazodone, nelfinavir, ritonavir, saquinavir, telithromycin, troleandomycin (TAO), voriconazole, or grapefruit or grapefruit juice] or when using concomitantly with an inhibitor of both CYP3A4 and CYP1A2 (e.g., ciprofloxacin). Avoid concomitant use if possible [see Drug Interactions (7)]. • When restarting therapy following withholding treatment for a dose-limiting toxicity that has resolved to baseline or grade ≤ 1.Increase TARCEVA by 50 mg increments as tolerated for:
• Concomitant use with CYP3A4 inducers, such as rifampin, rifabutin, rifapentine, phenytoin, carbamazepine, phenobarbital, or St. John’s Wort. Increase doses by 50 mg increments at 2 week intervals to a maximum of 450 mg. Avoid concomitant use, if possible [see Drug Interactions (7)]. • Concurrent cigarette smoking. Increase by 50 mg increments at 2 week intervals to a maximum of 300 mg. Immediately reduce the dose of TARCEVA to the recommended dose (150 mg or 100 mg daily) upon cessation of smoking [see Drug Interactions (7) and Clinical Pharmacology (12.3)].Drugs Affecting Gastric pH
• Avoid concomitant use of TARCEVA with proton pump inhibitors if possible. Separation of doses may not eliminate the interaction since proton pump inhibitors affect the pH of the upper GI tract for an extended period. • If treatment with an H 2-receptor antagonist such as ranitidine is required, TARCEVA must be taken 10 hours after the H 2-receptor antagonist dosing and at least 2 hours before the next dose of the H 2-receptor antagonist. • Although the effect of antacids on erlotinib pharmacokinetics has not been evaluated, the antacid dose and the TARCEVA dose should be separated by several hours, if an antacid is necessary.
Login To Your Free Account



![Tarceva (Erlotinib Hydrochloride) Tablet [Genentech, Inc.]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=57bccb29-1c47-4c64-ab6a-77960a91cc20&name=8c1ddd38-046e-46c6-9e57-cc6f0e0f4357-07.jpg)