FDA records indicate that there are no current recalls for this drug.
Are you a medical professional?
Trending Topics
Tegretol Recall
Get an alert when a recall is issued.
Questions & Answers
Side Effects & Adverse Reactions
Serious and sometimes fatal dermatologic reactions, including toxic epidermal necrolysis (TEN) and Stevens-Johnson syndrome (SJS), have been reported with Tegretol treatment. The risk of these events is estimated to be about 1 to 6 per 10,000 new users in countries with mainly Caucasian populations. However, the risk in some Asian countries is estimated to be about 10 times higher. Tegretol should be discontinued at the first sign of a rash, unless the rash is clearly not drug-related. If signs or symptoms suggest SJS/TEN, use of this drug should not be resumed and alternative therapy should be considered.
Retrospective case-control studies have found that in patients of Chinese ancestry there is a strong association between the risk of developing SJS/TEN with carbamazepine treatment and the presence of an inherited variant of the HLA-B gene, HLA-B*1502. The occurrence of higher rates of these reactions in countries with higher frequencies of this allele suggests that the risk may be increased in allele-positive individuals of any ethnicity.
Across Asian populations, notable variation exists in the prevalence of HLA-B*1502. Greater than 15% of the population is reported positive in Hong Kong, Thailand, Malaysia, and parts of the Philippines, compared to about 10% in Taiwan and 4% in North China. South Asians, including Indians, appear to have intermediate prevalence of HLA-B*1502, averaging 2% to 4%, but higher in some groups. HLA-B*1502 is present in less than 1% of the population in Japan and Korea.
HLA-B*1502 is largely absent in individuals not of Asian origin (e.g., Caucasians, African-Americans, Hispanics, and Native Americans).
Prior to initiating Tegretol therapy, testing for HLA-B*1502 should be performed in patients with ancestry in populations in which HLA-B*1502 may be present. In deciding which patients to screen, the rates provided above for the prevalence of HLA-B*1502 may offer a rough guide, keeping in mind the limitations of these figures due to wide variability in rates even within ethnic groups, the difficulty in ascertaining ethnic ancestry, and the likelihood of mixed ancestry. Tegretol should not be used in patients positive for HLA-B*1502 unless the benefits clearly outweigh the risks. Tested patients who are found to be negative for the allele are thought to have a low risk of SJS/TEN (see BOXED WARNING and PRECAUTIONS, Laboratory Tests).
Over 90% of Tegretol treated patients who will experience SJS/TEN have this reaction within the first few months of treatment. This information may be taken into consideration in determining the need for screening of genetically at-risk patients currently on Tegretol.
The HLA-B*1502 allele has not been found to predict risk of less severe adverse cutaneous reactions from Tegretol such as maculopapular eruption (MPE) or to predict Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS).
Limited evidence suggests that HLA-B*1502 may be a risk factor for the development of SJS/TEN in patients of Chinese ancestry taking other antiepileptic drugs associated with SJS/TEN, including phenytoin. Consideration should be given to avoiding use of other drugs associated with SJS/TEN in HLA-B*1502 positive patients, when alternative therapies are otherwise equally acceptable.
Retrospective case-control studies in patients of European, Korean, and Japanese ancestry have found a moderate association between the risk of developing hypersensitivity reactions and the presence of HLA-A*3101, an inherited allelic variant of the HLA-A gene, in patients using carbamazepine. These hypersensitivity reactions include SJS/TEN, maculopapular eruptions, and Drug Reaction with Eosinophilia and Systemic Symptoms (see DRESS/Multiorgan hypersensitivity below).
HLA-A*3101 is expected to be carried by more than 15% of patients of Japanese, Native American, Southern Indian (for example, Tamil Nadu) and some Arabic ancestry; up to about 10% in patients of Han Chinese, Korean, European, Latin American, and other Indian ancestry; and up to about 5% in African-Americans and patients of Thai, Taiwanese, and Chinese (Hong Kong) ancestry.
The risks and benefits of Tegretol therapy should be weighed before considering Tegretol in patients known to be positive for HLA-A*3101.
Application of HLA genotyping as a screening tool has important limitations and must never substitute for appropriate clinical vigilance and patient management. Many HLA-B*1502-positive and HLA-A*3101-positive patients treated with Tegretol will not develop SJS/TEN or other hypersensitivity reactions, and these reactions can still occur infrequently in HLA-B*1502-negative and HLA-A*3101-negative patients of any ethnicity. The role of other possible factors in the development of, and morbidity from, SJS/TEN and other hypersensitivity reactions, such as antiepileptic drug (AED) dose, compliance, concomitant medications, comorbidities, and the level of dermatologic monitoring, have not been studied.
Aplastic anemia and agranulocytosis have been reported in association with the use of TEGRETOL (see BOXED WARNING). Patients with a history of adverse hematologic reaction to any drug may be particularly at risk of bone marrow depression.
Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS), also known as multiorgan hypersensitivity, has occurred with Tegretol. Some of these events have been fatal or life-threatening. DRESS typically, although not exclusively, presents with fever, rash, and/or lymphadenopathy, in association with other organ system involvement, such as hepatitis, nephritis, hematologic abnormalities, myocarditis, or myositis sometimes resembling an acute viral infection. Eosinophilia is often present. This disorder is variable in its expression, and other organ systems not noted here may be involved. It is important to note that early manifestations of hypersensitivity (e.g., fever, lymphadenopathy) may be present even though rash is not evident. If such signs or symptoms are present, the patient should be evaluated immediately. Tegretol should be discontinued if an alternative etiology for the signs or symptoms cannot be established.
Hypersensitivity
Hypersensitivity reactions to carbamazepine have been reported in patients who previously experienced this reaction to anticonvulsants including phenytoin, primidone, and phenobarbital. If such history is present, benefits and risks should be carefully considered and, if carbamazepine is initiated, the signs and symptoms of hypersensitivity should be carefully monitored.
In patients who have exhibited hypersensitivity reactions to carbamazepine, approximately 25 to 30% may experience hypersensitivity reactions with oxcarbazepine (Trileptal®).
Antiepileptic drugs (AEDs), including Tegretol, increase the risk of suicidal thoughts or behavior in patients taking these drugs for any indication. Patients treated with any AED for any indication should be monitored for the emergence or worsening of depression, suicidal thoughts or behavior, and/or any unusual changes in mood or behavior.
Pooled analyses of 199 placebo-controlled clinical trials (mono- and adjunctive therapy) of 11 different AEDs showed that patients randomized to one of the AEDs had approximately twice the risk (adjusted Relative Risk 1.8, 95% CI:1.2, 2.7) of suicidal thinking or behavior compared to patients randomized to placebo. In these trials, which had a median treatment duration of 12 weeks, the estimated incidence rate of suicidal behavior or ideation among 27,863 AED-treated patients was 0.43%, compared to 0.24% among 16,029 placebo-treated patients, representing an increase of approximately one case of suicidal thinking or behavior for every 530 patients treated. There were four suicides in drug-treated patients in the trials and none in placebo-treated patients, but the number is too small to allow any conclusion about drug effect on suicide.
The increased risk of suicidal thoughts or behavior with AEDs was observed as early as one week after starting drug treatment with AEDs and persisted for the duration of treatment assessed. Because most trials included in the analysis did not extend beyond 24 weeks, the risk of suicidal thoughts or behavior beyond 24 weeks could not be assessed.
The risk of suicidal thoughts or behavior was generally consistent among drugs in the data analyzed. The finding of increased risk with AEDs of varying mechanisms of action and across a range of indications suggests that the risk applies to all AEDs used for any indication. The risk did not vary substantially by age (5 to 100 years) in the clinical trials analyzed. Table 1 shows absolute and relative risk by indication for all evaluated AEDs.
| Indication | Placebo Patients with Events Per 1,000 Patients |
Drug Patients with Events Per 1,000 Patients | Relative Risk: Incidence of Events in Drug Patients/Incidence in Placebo Patients | Risk Difference: Additional Drug Patients with Events Per 1,000 Patients |
| Epilepsy | 1.0 | 3.4 | 3.5 | 2.4 |
| Psychiatric | 5.7 | 8.5 | 1.5 | 2.9 |
| Other | 1.0 | 1.8 | 1.9 | 0.9 |
| Total | 2.4 | 4.3 | 1.8 | 1.9 |
The relative risk for suicidal thoughts or behavior was higher in clinical trials for epilepsy than in clinical trials for psychiatric or other conditions, but the absolute risk differences were similar for the epilepsy and psychiatric indications.
Anyone considering prescribing Tegretol or any other AED must balance the risk of suicidal thoughts or behavior with the risk of untreated illness. Epilepsy and many other illnesses for which AEDs are prescribed are themselves associated with morbidity and mortality and an increased risk of suicidal thoughts and behavior. Should suicidal thoughts and behavior emerge during treatment, the prescriber needs to consider whether the emergence of these symptoms in any given patient may be related to the illness being treated.
Patients, their caregivers, and families should be informed that AEDs increase the risk of suicidal thoughts and behavior and should be advised of the need to be alert for the emergence or worsening of the signs and symptoms of depression, any unusual changes in mood or behavior, or the emergence of suicidal thoughts, behavior, or thoughts about self-harm. Behaviors of concern should be reported immediately to healthcare providers.
Tegretol has shown mild anticholinergic activity; therefore, patients with increased intraocular pressure should be closely observed during therapy.
Because of the relationship of the drug to other tricyclic compounds, the possibility of activation of a latent psychosis and, in elderly patients, of confusion or agitation should be borne in mind.
The use of Tegretol should be avoided in patients with a history of hepatic porphyria (e.g., acute intermittent porphyria, variegate porphyria, porphyria cutanea tarda). Acute attacks have been reported in such patients receiving Tegretol therapy. Carbamazepine administration has also been demonstrated to increase porphyrin precursors in rodents, a presumed mechanism for the induction of acute attacks of porphyria.
As with all antiepileptic drugs, Tegretol should be withdrawn gradually to minimize the potential of increased seizure frequency.
Carbamazepine can cause fetal harm when administered to a pregnant woman.
Epidemiological data suggest that there may be an association between the use of carbamazepine during pregnancy and congenital malformations, including spina bifida. There have also been reports that associate carbamazepine with developmental disorders and congenital anomalies (e.g., craniofacial defects, cardiovascular malformations, hypospadias, and anomalies involving various body systems). Developmental delays based on neurobehavioral assessments have been reported. When treating or counseling women of childbearing potential, the prescribing physician will wish to weigh the benefits of therapy against the risks. If this drug is used during pregnancy, or if the patient becomes pregnant while taking this drug, the patient should be apprised of the potential hazard to the fetus.
Retrospective case reviews suggest that, compared with monotherapy, there may be a higher prevalence of teratogenic effects associated with the use of anticonvulsants in combination therapy. Therefore, if therapy is to be continued, monotherapy may be preferable for pregnant women.
In humans, transplacental passage of carbamazepine is rapid (30 to 60 minutes), and the drug is accumulated in the fetal tissues, with higher levels found in liver and kidney than in brain and lung.
Carbamazepine has been shown to have adverse effects in reproduction studies in rats when given orally in dosages 10 to 25 times the maximum human daily dosage (MHDD) of 1200 mg on a mg/kg basis or 1.5 to 4 times the MHDD on a mg/m2 basis. In rat teratology studies, 2 of 135 offspring showed kinked ribs at 250 mg/kg and 4 of 119 offspring at 650 mg/kg showed other anomalies (cleft palate, 1; talipes, 1; anophthalmos, 2). In reproduction studies in rats, nursing offspring demonstrated a lack of weight gain and an unkempt appearance at a maternal dosage level of 200 mg/kg.
Antiepileptic drugs should not be discontinued abruptly in patients in whom the drug is administered to prevent major seizures because of the strong possibility of precipitating status epilepticus with attendant hypoxia and threat to life. In individual cases where the severity and frequency of the seizure disorder are such that removal of medication does not pose a serious threat to the patient, discontinuation of the drug may be considered prior to and during pregnancy, although it cannot be said with any confidence that even minor seizures do not pose some hazard to the developing embryo or fetus.
Tests to detect defects using currently accepted procedures should be considered a part of routine prenatal care in childbearing women receiving carbamazepine.
There have been a few cases of neonatal seizures and/or respiratory depression associated with maternal Tegretol and other concomitant anticonvulsant drug use. A few cases of neonatal vomiting, diarrhea, and/or decreased feeding have also been reported in association with maternal Tegretol use. These symptoms may represent a neonatal withdrawal syndrome.
To provide information regarding the effects of in utero exposure to Tegretol, physicians are advised to recommend that pregnant patients taking Tegretol enroll in the North American Antiepileptic Drug (NAAED) Pregnancy Registry. This can be done by calling the toll free number 1-888-233-2334, and must be done by patients themselves. Information on the registry can also be found at the website http://www.aedpregnancyregistry.org/.
Legal Issues
There is currently no legal information available for this drug.
FDA Safety Alerts
There are currently no FDA safety alerts available for this drug.
Manufacturer Warnings
There is currently no manufacturer warning information available for this drug.
FDA Labeling Changes
There are currently no FDA labeling changes available for this drug.
Uses
Tegretol is indicated for use as an anticonvulsant drug. Evidence supporting efficacy of Tegretol as an anticonvulsant was derived from active drug-controlled studies that enrolled patients with the following seizure types:
- Partial seizures with complex symptomatology (psychomotor, temporal lobe). Patients with these seizures appear to show greater improvement than those with other types.
- Generalized tonic-clonic seizures (grand mal).
- Mixed seizure patterns which include the above, or other partial or generalized seizures. Absence seizures (petit mal) do not appear to be controlled by Tegretol (see PRECAUTIONS, General).
Tegretol is indicated in the treatment of the pain associated with true trigeminal neuralgia.
Beneficial results have also been reported in glossopharyngeal neuralgia.
This drug is not a simple analgesic and should not be used for the relief of trivial aches or pains.
History
There is currently no drug history available for this drug.
Other Information
Tegretol, carbamazepine USP, is an anticonvulsant and specific analgesic for trigeminal neuralgia, available for oral administration as chewable tablets of 100 mg, tablets of 200 mg, XR tablets of 100, 200, and 400 mg, and as a suspension of 100 mg/5 mL (teaspoon). Its chemical name is 5H-dibenz[b,f ]azepine-5-carboxamide, and its structural formula is:

Carbamazepine USP is a white to off-white powder, practically insoluble in water and soluble in alcohol and in acetone. Its molecular weight is 236.27.
Inactive Ingredients Tablets: Colloidal silicon dioxide, D&C Red No. 30 Aluminum Lake (chewable tablets only), FD&C Red No. 40 (200 mg tablets only), flavoring (chewable tablets only), gelatin, glycerin, magnesium stearate, sodium starch glycolate (chewable tablets only), starch, stearic acid, and sucrose (chewable tablets only). Suspension: Citric acid, FD&C Yellow No. 6, flavoring, polymer, potassium sorbate, propylene glycol, purified water, sorbitol, sucrose, and xanthan gum. Tegretol-XR tablets: cellulose compounds, dextrates, iron oxides, magnesium stearate, mannitol, polyethylene glycol, sodium lauryl sulfate, titanium dioxide (200 mg tablets only).
Sources
Tegretol Manufacturers
-
Novartis Pharmaceuticals Corporation
![Tegretol (Carbamazepine) Tablet, Chewable Tegretol (Carbamazepine) Suspension Tegretol (Carbamazepine) Tablet Tegretol Xr (Carbamazepine) Tablet, Extended Release [Novartis Pharmaceuticals Corporation]](/wp-content/themes/bootstrap/assets/img/loading2.gif)
Tegretol | Novartis Pharmaceuticals Corporation
![Tegretol (Carbamazepine) Tablet, Chewable Tegretol (Carbamazepine) Suspension Tegretol (Carbamazepine) Tablet Tegretol Xr (Carbamazepine) Tablet, Extended Release [Novartis Pharmaceuticals Corporation] Tegretol (Carbamazepine) Tablet, Chewable Tegretol (Carbamazepine) Suspension Tegretol (Carbamazepine) Tablet Tegretol Xr (Carbamazepine) Tablet, Extended Release [Novartis Pharmaceuticals Corporation]](/wp-content/themes/bootstrap/assets/img/loading2.gif)
Tegretol suspension in combination with liquid chlorpromazine or thioridazine results in precipitate formation, and, in the case of chlorpromazine, there has been a report of a patient passing an orange rubbery precipitate in the stool following coadministration of the two drugs (see PRECAUTIONS, Drug Interactions). Because the extent to which this occurs with other liquid medications is not known, Tegretol suspension should not be administered simultaneously with other liquid medications or diluents.
Monitoring of blood levels has increased the efficacy and safety of anticonvulsants (see PRECAUTIONS, Laboratory Tests). Dosage should be adjusted to the needs of the individual patient. A low initial daily dosage with a gradual increase is advised. As soon as adequate control is achieved, the dosage may be reduced very gradually to the minimum effective level. Medication should be taken with meals.
Since a given dose of Tegretol suspension will produce higher peak levels than the same dose given as the tablet, it is recommended to start with low doses (children 6 to 12 years: ½ teaspoon q.i.d.) and to increase slowly to avoid unwanted side effects.
Conversion of patients from oral Tegretol tablets to Tegretol suspension: Patients should be converted by administering the same number of mg per day in smaller, more frequent doses (i.e., b.i.d. tablets to t.i.d. suspension).
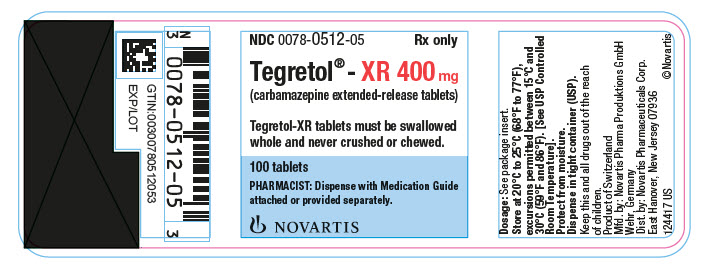
Tegretol-XR is an extended-release formulation for twice-a-day administration. When converting patients from Tegretol conventional tablets to Tegretol-XR, the same total daily mg dose of Tegretol-XR should be administered. Tegretol-XR tablets must be swallowed whole and never crushed or chewed. Tegretol-XR tablets should be inspected for chips or cracks. Damaged tablets, or tablets without a release portal, should not be consumed. Tegretol-XR tablet coating is not absorbed and is excreted in the feces; these coatings may be noticeable in the stool.
Epilepsy (SEE INDICATIONS AND USAGE)
Adults and children over 12 years of age-Initial: Either 200 mg b.i.d. for tablets and XR tablets, or 1 teaspoon q.i.d. for suspension (400 mg/day). Increase at weekly intervals by adding up to 200 mg/day using a b.i.d. regimen of Tegretol-XR or a t.i.d. or q.i.d. regimen of the other formulations until the optimal response is obtained. Dosage generally should not exceed 1000 mg daily in children 12 to 15 years of age, and 1200 mg daily in patients above 15 years of age. Doses up to 1600 mg daily have been used in adults in rare instances. Maintenance: Adjust dosage to the minimum effective level, usually 800 to 1200 mg daily.
Children 6 to 12 years of age-Initial: Either 100 mg b.i.d. for tablets or XR tablets, or ½ teaspoon q.i.d. for suspension (200 mg/day). Increase at weekly intervals by adding up to 100 mg/day using a b.i.d. regimen of Tegretol-XR or a t.i.d. or q.i.d. regimen of the other formulations until the optimal response is obtained. Dosage generally should not exceed 1000 mg daily. Maintenance: Adjust dosage to the minimum effective level, usually 400 to 800 mg daily.
Children under 6 years of age-Initial: 10 to 20 mg/kg/day b.i.d. or t.i.d. as tablets, or q.i.d. as suspension. Increase weekly to achieve optimal clinical response administered t.i.d. or q.i.d. Maintenance: Ordinarily, optimal clinical response is achieved at daily doses below 35 mg/kg. If satisfactory clinical response has not been achieved, plasma levels should be measured to determine whether or not they are in the therapeutic range. No recommendation regarding the safety of carbamazepine for use at doses above 35 mg/kg/24 hours can be made.
Combination Therapy: Tegretol may be used alone or with other anticonvulsants. When added to existing anticonvulsant therapy, the drug should be added gradually while the other anticonvulsants are maintained or gradually decreased, except phenytoin, which may have to be increased (see PRECAUTIONS, Drug Interactions, and Pregnancy Category D).
Trigeminal Neuralgia (SEE INDICATIONS AND USAGE)
Initial: On the first day, either 100 mg b.i.d. for tablets or XR tablets, or ½ teaspoon q.i.d. for suspension, for a total daily dose of 200 mg. This daily dose may be increased by up to 200 mg/day using increments of 100 mg every 12 hours for tablets or XR tablets, or 50 mg (½ teaspoon) q.i.d. for suspension, only as needed to achieve freedom from pain. Do not exceed 1200 mg daily. Maintenance: Control of pain can be maintained in most patients with 400 to 800 mg daily. However, some patients may be maintained on as little as 200 mg daily, while others may require as much as 1200 mg daily. At least once every 3 months throughout the treatment period, attempts should be made to reduce the dose to the minimum effective level or even to discontinue the drug.
Dosage Information Initial Dose Subsequent Dose Maximum Daily Dose Indication Tablet* XR† Suspension Tablet* XR† Suspension Tablet* XR† Suspension Epilepsy
Under 6 yr 10-20 mg/kg/day
b.i.d. or t.i.d. 10-20 mg/kg/day
q.i.d. Increase weekly to achieve optimal clinical response, t.i.d. or q.i.d. Increase weekly to achieve optimal clinical response, t.i.d. or q.i.d. 35 mg/kg/24 hr (see Dosage and Administration section above) 35 mg/kg/24 hr (see Dosage and Administration section above) 6-12 yr 100 mg b.i.d.
(200 mg/day) 100 mg b.i.d.
(200 mg/day) ½ tsp q.i.d.
(200 mg/day) Add up to 100 mg/day at weekly intervals, t.i.d. or q.i.d. Add 100 mg/day at weekly intervals, b.i.d. Add up to 1 tsp (100 mg)/day at weekly intervals, t.i.d. or q.i.d. 1000 mg/24 hr Over 12 yr 200 mg b.i.d.
(400 mg/day) 200 mg b.i.d.
(400 mg/day) 1 tsp q.i.d.
(400 mg/day) Add up to 200 mg/day at weekly intervals, t.i.d. or q.i.d. Add up to 200 mg/day at weekly intervals, b.i.d. Add up to 2 tsp (200 mg)/day at weekly intervals, t.i.d. or q.i.d. 1000 mg/24 hr (12-15 yr)
1200 mg/24 hr (>15 yr)
1600 mg/24 hr (adults, in rare instances) Trigeminal Neuralgia 100 mg b.i.d.
(200 mg/day) 100 mg b.i.d.
(200 mg/day) ½ tsp q.i.d.
(200 mg/day) Add up to 200 mg/day in increments of 100 mg every 12 hr Add up to 200 mg/day in increments of 100 mg every 12 hr Add up to 2 tsp (200 mg)/day
in increments of 50 mg
(½ tsp) q.i.d. 1200 mg/24 hr*Tablet = Chewable or conventional tablets
†XR = Tegretol-XR extended-release tablets
-
Remedyrepack Inc.
![Tegretol (Carbamazepine) Tablet [Remedyrepack Inc. ]](/wp-content/themes/bootstrap/assets/img/loading2.gif)
Tegretol | Remedyrepack Inc.
![Tegretol (Carbamazepine) Tablet [Remedyrepack Inc. ] Tegretol (Carbamazepine) Tablet [Remedyrepack Inc. ]](/wp-content/themes/bootstrap/assets/img/loading2.gif)
Tegretol suspension in combination with liquid chlorpromazine or thioridazine results in precipitate formation, and, in the case of chlorpromazine, there has been a report of a patient passing an orange rubbery precipitate in the stool following coadministration of the two drugs (see PRECAUTIONS, Drug Interactions). Because the extent to which this occurs with other liquid medications is not known, Tegretol suspension should not be administered simultaneously with other liquid medications or diluents.
Monitoring of blood levels has increased the efficacy and safety of anticonvulsants (see PRECAUTIONS, Laboratory Tests). Dosage should be adjusted to the needs of the individual patient. A low initial daily dosage with a gradual increase is advised. As soon as adequate control is achieved, the dosage may be reduced very gradually to the minimum effective level. Medication should be taken with meals.
Since a given dose of Tegretol suspension will produce higher peak levels than the same dose given as the tablet, it is recommended to start with low doses (children 6 to 12 years: ½ teaspoon q.i.d.) and to increase slowly to avoid unwanted side effects.
Conversion of patients from oral Tegretol tablets to Tegretol suspension: Patients should be converted by administering the same number of mg per day in smaller, more frequent doses (i.e., b.i.d. tablets to t.i.d. suspension).
Tegretol-XR is an extended-release formulation for twice-a-day administration. When converting patients from Tegretol conventional tablets to Tegretol-XR, the same total daily mg dose of Tegretol-XR should be administered. Tegretol-XR tablets must be swallowed whole and never crushed or chewed. Tegretol-XR tablets should be inspected for chips or cracks. Damaged tablets, or tablets without a release portal, should not be consumed. Tegretol-XR tablet coating is not absorbed and is excreted in the feces; these coatings may be noticeable in the stool.
Epilepsy (SEE INDICATIONS AND USAGE)
Adults and children over 12 years of age-Initial: Either 200 mg b.i.d. for tablets and XR tablets, or 1 teaspoon q.i.d. for suspension (400 mg/day). Increase at weekly intervals by adding up to 200 mg/day using a b.i.d. regimen of Tegretol-XR or a t.i.d. or q.i.d. regimen of the other formulations until the optimal response is obtained. Dosage generally should not exceed 1000 mg daily in children 12 to 15 years of age, and 1200 mg daily in patients above 15 years of age. Doses up to 1600 mg daily have been used in adults in rare instances. Maintenance: Adjust dosage to the minimum effective level, usually 800 to 1200 mg daily.
Children 6 to 12 years of age-Initial: Either 100 mg b.i.d. for tablets or XR tablets, or ½ teaspoon q.i.d. for suspension (200 mg/day). Increase at weekly intervals by adding up to 100 mg/day using a b.i.d. regimen of Tegretol-XR or a t.i.d. or q.i.d. regimen of the other formulations until the optimal response is obtained. Dosage generally should not exceed 1000 mg daily. Maintenance: Adjust dosage to the minimum effective level, usually 400 to 800 mg daily.
Children under 6 years of age-Initial: 10 to 20 mg/kg/day b.i.d. or t.i.d. as tablets, or q.i.d. as suspension. Increase weekly to achieve optimal clinical response administered t.i.d. or q.i.d. Maintenance: Ordinarily, optimal clinical response is achieved at daily doses below 35 mg/kg. If satisfactory clinical response has not been achieved, plasma levels should be measured to determine whether or not they are in the therapeutic range. No recommendation regarding the safety of carbamazepine for use at doses above 35 mg/kg/24 hours can be made.
Combination Therapy: Tegretol may be used alone or with other anticonvulsants. When added to existing anticonvulsant therapy, the drug should be added gradually while the other anticonvulsants are maintained or gradually decreased, except phenytoin, which may have to be increased (see PRECAUTIONS, Drug Interactions, and Pregnancy Category D).
Trigeminal Neuralgia (SEE INDICATIONS AND USAGE)
Initial: On the first day, either 100 mg b.i.d. for tablets or XR tablets, or ½ teaspoon q.i.d. for suspension, for a total daily dose of 200 mg. This daily dose may be increased by up to 200 mg/day using increments of 100 mg every 12 hours for tablets or XR tablets, or 50 mg (½ teaspoon) q.i.d. for suspension, only as needed to achieve freedom from pain. Do not exceed 1200 mg daily. Maintenance: Control of pain can be maintained in most patients with 400 to 800 mg daily. However, some patients may be maintained on as little as 200 mg daily, while others may require as much as 1200 mg daily. At least once every 3 months throughout the treatment period, attempts should be made to reduce the dose to the minimum effective level or even to discontinue the drug.
Dosage Information Initial Dose Subsequent Dose Maximum Daily Dose Indication Tablet* XR† Suspension Tablet* XR† Suspension Tablet* XR† Suspension Epilepsy
Under 6 yr 10-20 mg/kg/day
b.i.d. or t.i.d. 10-20 mg/kg/day
q.i.d. Increase weekly to achieve optimal clinical response, t.i.d. or q.i.d. Increase weekly to achieve optimal clinical response, t.i.d. or q.i.d. 35 mg/kg/24 hr (see Dosage and Administration section above) 35 mg/kg/24 hr (see Dosage and Administration section above) 6-12 yr 100 mg b.i.d.
(200 mg/day) 100 mg b.i.d.
(200 mg/day) ½ tsp q.i.d.
(200 mg/day) Add up to 100 mg/day at weekly intervals, t.i.d. or q.i.d. Add 100 mg/day at weekly intervals, b.i.d. Add up to 1 tsp (100 mg)/day at weekly intervals, t.i.d. or q.i.d. 1000 mg/24 hr Over 12 yr 200 mg b.i.d.
(400 mg/day) 200 mg b.i.d.
(400 mg/day) 1 tsp q.i.d.
(400 mg/day) Add up to 200 mg/day at weekly intervals, t.i.d. or q.i.d. Add up to 200 mg/day at weekly intervals, b.i.d. Add up to 2 tsp (200 mg)/day at weekly intervals, t.i.d. or q.i.d. 1000 mg/24 hr (12-15 yr)
1200 mg/24 hr (>15 yr)
1600 mg/24 hr (adults, in rare instances) Trigeminal Neuralgia 100 mg b.i.d.
(200 mg/day) 100 mg b.i.d.
(200 mg/day) ½ tsp q.i.d.
(200 mg/day) Add up to 200 mg/day in increments of 100 mg every 12 hr Add up to 200 mg/day in increments of 100 mg every 12 hr Add up to 2 tsp (200 mg)/day
in increments of 50 mg
(½ tsp) q.i.d. 1200 mg/24 hr*Tablet = Chewable or conventional tablets
†XR = Tegretol-XR extended-release tablets
Login To Your Free Account


![Tegretol (Carbamazepine) Tablet [Remedyrepack Inc. ]](https://www.recallguide.org/wp-content/themes/bootstrap/assets/img/drug-image-placeholder.jpg)