Thyrotox Recall
Get an alert when a recall is issued.
Questions & Answers
Side Effects & Adverse Reactions
There is currently no warning information available for this product. We apologize for any inconvenience.
Legal Issues
There is currently no legal information available for this drug.
FDA Safety Alerts
There are currently no FDA safety alerts available for this drug.
Manufacturer Warnings
There is currently no manufacturer warning information available for this drug.
FDA Labeling Changes
There are currently no FDA labeling changes available for this drug.
Uses
Sildenafil injection is indicated for the treatment of pulmonary arterial hypertension (WHO Group I) in adults to improve exercise ability and delay clinical worsening. The delay in clinical worsening was demonstrated when sildenafil injection was added to background epoprostenol therapy [see Clinical Studies (14)].
Studies establishing effectiveness were short-term (12 to 16 weeks), and included predominately patients with New York Heart Association (NYHA) Functional Class II-III symptoms and idiopathic etiology (71%) or associated with connective tissue disease (CTD) (25%).
History
There is currently no drug history available for this drug.
Other Information
Sildenafil injection, phosphodiesterase-5 (PDE-5) inhibitor, is the citrate salt of sildenafil, a selective inhibitor of cyclic guanosine monophosphate (cGMP)-specific phosphodiesterase type-5 (PDE-5). Sildenafil is also marketed as VIAGRA® for erectile dysfunction.
Sildenafil citrate is designated chemically as 1-[[3-(6,7-dihydro-1-methyl-7-oxo-3-propyl-1H-pyrazolo [4,3-d] pyrimidin-5-yl)-4-ethoxyphenyl] sulfonyl]-4-methylpiperazine citrate and has the following structural formula:
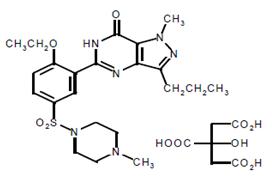
Sildenafil citrate USP is a white or almost white slightly hygroscopic crystalline powder with a solubility of 3.5 mg/mL in water and a molecular weight of 666.7.
Sildenafil injection is supplied as a clear, colorless, sterile, ready to use solution in a single-use vial containing 10 mg/12.5 mL of sildenafil. Each mL of solution contains 1.124 mg sildenafil citrate USP equivalent to 0.8 mg sildenafil, 50.5 mg dextrose and water for injection.
Sources