FDA records indicate that there are no current recalls for this drug.
Are you a medical professional?
Trending Topics
Thyrotropin Releasing Hormone Trh Recall
Get an alert when a recall is issued.
Questions & Answers
Side Effects & Adverse Reactions
Transient changes in blood pressure, either increases or decreases, frequently occur immediately following administration of TRH. Blood pressure should therefore be measured before TRH is administered and at frequent intervals during the first 15 minutes after its administration.
Increases in systolic pressure (usually less than 30 mm Hg) and/or increases in diastolic pressure (usually less than 20 mm Hg) have been observed more frequently than decreases in pressure. These changes have not ordinarily persisted for more than 15 minutes nor have they required therapy. More severe degrees of hypertension or hypotension with or without syncope have been reported in a few patients. To minimize the incidence and/or severity of hypotension, the patient should be supine before, during, and after TRH administration. If a clinically important change in blood pressure occurs, monitoring of blood pressure should be continued until it returns to base-line levels.
TRH should not be administered to patients in whom marked, rapid changes in blood pressure would be dangerous unless the potential benefit clearly outweighs the potential risk
Legal Issues
There is currently no legal information available for this drug.
FDA Safety Alerts
There are currently no FDA safety alerts available for this drug.
Manufacturer Warnings
There is currently no manufacturer warning information available for this drug.
FDA Labeling Changes
There are currently no FDA labeling changes available for this drug.
Uses
TRH is indicated as an adjunctive agent in the diagnostic assessment of thyroid function. As an adjunct to other diagnostic procedures, testing with TRH (protirelin) may yield useful information in patients with pituitary or hypothalamic dysfunction.
TRH is indicated as an adjunct to evaluate the effectiveness of thyrotropin suppression with a particular dose of T4 in patients with nodular or diffuse goiter. A normal TSH baseline value and a minimal difference between the 30 minute and baseline response to TRH injection would indicate adequate suppression of the pituitary secretion of TSH.
TRH may be used, adjunctively, for adjustment of thyroid hormone dosage given to patients with primary hypothyroidism. A normal or slightly blunted TSH response, thirty minutes following TRH injection, would indicate adequate replacement therapy.
History
There is currently no drug history available for this drug.
Other Information
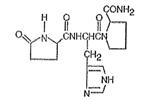
Chemically, TRH (protirelin) is identified as 5-oxo-L-prolyl-L-histidyl-L-proline amide. It is a synthetic tripeptide that is believed to be structurally identical to the naturally-occurring thyrotropin-releasing hormone produced by the hypothalamus. The CAS Registry Number is 24305-27-9. The structural formula is:
Figure 1

TRH is supplied as a solution of 1 mL in a 5 mL vial. Each vial contains 500 mcg protirelin, 1.8 mg Methylparaben, 0.2 mg Propylparaben, and 9.0 mg Sodium Chloride. TRH is intended for intravenous administration following dilution with 1 mL sterile water for injection.
Sources
Thyrotropin Releasing Hormone Trh Manufacturers
-
Anazaohealth Corporation
![Thyrotropin Releasing Hormone Trh (Thyrotropin Releasing Hormone) Solution [Anazaohealth Corporation]](/wp-content/themes/bootstrap/assets/img/loading2.gif)
Thyrotropin Releasing Hormone Trh | Anazaohealth Corporation
![Thyrotropin Releasing Hormone Trh (Thyrotropin Releasing Hormone) Solution [Anazaohealth Corporation] Thyrotropin Releasing Hormone Trh (Thyrotropin Releasing Hormone) Solution [Anazaohealth Corporation]](/wp-content/themes/bootstrap/assets/img/loading2.gif)
TRH is intended for intravenous administration with the patient in the supine position. The drug is administered as a bolus over a period of 15 to 30 seconds, with the patient remaining supine until all scheduled post injection blood samples have been taken. Blood pressure should be measured before TRH is administered and at frequent intervals during the first 15 minutes thereafter (see WARNINGS). Have the patient urinate before injecting TRH.
Adults: 500 μg. Doses between 200 and 500 μg have been used. 500 μg is considered the optimum dose to give the maximum response in the greatest number of patients. Doses greater than 500 μg are unlikely to elicit a greater TSH response.
Children age 6 to 16 years: 7 μg/kg body weight up to a dose of 500 μg.
Infants and children up to 6 years: Experience is limited in this age group; doses of 7μg/kg have been administered.
One blood sample for TSH assay should be drawn immediately prior to the injection of TRH, and a second sample should be obtained 30 minutes after injection.
The TSH response to TRH is reduced by repetitive administration of the drug. Accordingly, if the TRH test is repeated, an interval of seven days before testing is recommended.
Elevated serum lipids may interfere with the TSH assay. Thus, fasting (except in patients with hypopituitarism) or a low-fat meal is recommended prior to the test.
Login To Your Free Account