FDA records indicate that there are no current recalls for this drug.
Are you a medical professional?
Trending Topics
Torisel Recall
Get an alert when a recall is issued.
Questions & Answers
Side Effects & Adverse Reactions
There is currently no warning information available for this product. We apologize for any inconvenience.
Legal Issues
There is currently no legal information available for this drug.
FDA Safety Alerts
There are currently no FDA safety alerts available for this drug.
Manufacturer Warnings
There is currently no manufacturer warning information available for this drug.
FDA Labeling Changes
There are currently no FDA labeling changes available for this drug.
Uses
TORISEL is indicated for the treatment of advanced renal cell carcinoma.
History
There is currently no drug history available for this drug.
Other Information
Temsirolimus, an inhibitor of mTOR, is an antineoplastic agent.
Temsirolimus is a white to off-white powder with a molecular formula of C56H87NO16 and a molecular weight of 1030.30. It is non-hygroscopic. Temsirolimus is practically insoluble in water and soluble in alcohol. It has no ionizable functional groups, and its solubility is independent of pH.
The chemical name of temsirolimus is (3S,6R,7E,9R,10R,12R,14S,15E,17E,19E,21S,23S,26R,27R,34aS)-9,10,12,13,14,21,22,23,24,25,26,27,32,33,34,34a-Hexadecahydro-9,27-dihydroxy-3-[(1R)-2-[(1S,3R,4R)-4-hydroxy-3-methoxycyclohexyl]-1-methylethyl]-10,21-dimethoxy-6,8,12,14,20,26-hexamethyl-23, 27-epoxy-3H-pyrido[2,1-c][1,4]oxaazacyclohentriacontine-1,5,11,28,29(4H,6H,31H)-pentone 4'-[2,2-bis(hydroxymethyl)propionate]; or Rapamycin, 42-[3-hydroxy-2-(hydroxymethyl)-2-methylpropanoate].

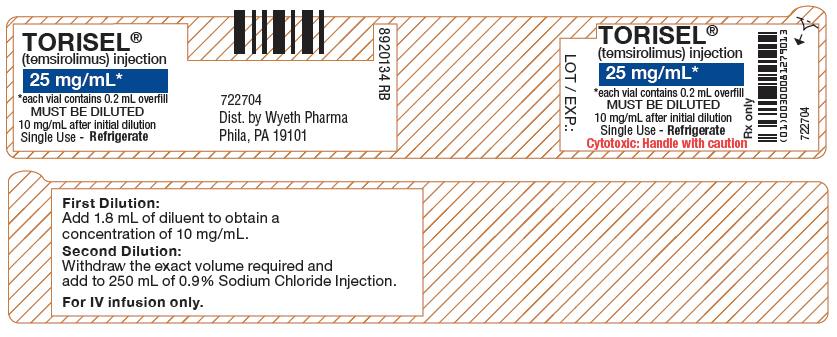
TORISEL (temsirolimus) injection, 25 mg/mL, is a clear, colorless to light yellow, non-aqueous, ethanolic, sterile solution. TORISEL (temsirolimus) injection requires two dilutions prior to intravenous infusion. TORISEL (temsirolimus) injection should be diluted only with the supplied DILUENT for TORISEL.
DILUENT for TORISEL is a sterile, non-aqueous solution that is supplied with TORISEL injection, as a kit.
TORISEL (temsirolimus) injection, 25 mg/mL:
Active ingredient: temsirolimus (25 mg/mL)
Inactive ingredients: dehydrated alcohol (39.5% w/v), dl-alpha-tocopherol (0.075% w/v), propylene glycol (50.3% w/v), and anhydrous citric acid (0.0025% w/v).
DILUENT for TORISEL:
Inactive ingredients: polysorbate 80 (40.0% w/v), polyethylene glycol 400 (42.8% w/v) and dehydrated alcohol (19.9% w/v).
After the TORISEL (temsirolimus) injection vial has been diluted with DILUENT for TORISEL, in accordance with the instructions in section 2.5, the solution contains 35.2% alcohol.
TORISEL (temsirolimus) injection and DILUENT for TORISEL are filled in clear glass vials with butyl rubber stoppers.
Sources
Torisel Manufacturers
-
Wyeth Pharmaceuticals Inc., A Subsidiary Of Pfizer Inc.
![Torisel (Temsirolimus) Kit [Wyeth Pharmaceuticals Inc., A Subsidiary Of Pfizer Inc.]](/wp-content/themes/bootstrap/assets/img/loading2.gif)
Torisel | Wyeth Pharmaceuticals Inc., A Subsidiary Of Pfizer Inc.
![Torisel (Temsirolimus) Kit [Wyeth Pharmaceuticals Inc., A Subsidiary Of Pfizer Inc.] Torisel (Temsirolimus) Kit [Wyeth Pharmaceuticals Inc., A Subsidiary Of Pfizer Inc.]](/wp-content/themes/bootstrap/assets/img/loading2.gif)
2.1 Advanced Renal Cell CarcinomaThe recommended dose of TORISEL for advanced renal cell carcinoma is 25 mg infused over a 30 – 60 minute period once a week.
Treatment should continue until disease progression or unacceptable toxicity occurs.
2.2 PremedicationPatients should receive prophylactic intravenous diphenhydramine 25 to 50 mg (or similar antihistamine) approximately 30 minutes before the start of each dose of TORISEL [see Warnings and Precautions (5.1)].
2.3 Dosage Interruption/AdjustmentTORISEL should be held for absolute neutrophil count (ANC) <1,000/mm3, platelet count <75,000/mm3, or NCI CTCAE grade 3 or greater adverse reactions. Once toxicities have resolved to grade 2 or less, TORISEL may be restarted with the dose reduced by 5 mg/week to a dose no lower than 15 mg/week.
2.4 Dose Modification GuidelinesHepatic Impairment: Use caution when treating patients with hepatic impairment. If TORISEL must be given in patients with mild hepatic impairment (bilirubin >1 – 1.5×ULN or AST >ULN but bilirubin ≤ULN), reduce the dose of TORISEL to 15 mg/week. TORISEL is contraindicated in patients with bilirubin >1.5×ULN [see Contraindications (4), Warnings and Precautions (5.2) and Use in Specific Populations (8.7)].
Concomitant Strong CYP3A4 Inhibitors: The concomitant use of strong CYP3A4 inhibitors should be avoided (e.g. ketoconazole, itraconazole, clarithromycin, atazanavir, indinavir, nefazodone, nelfinavir, ritonavir, saquinavir, telithromycin, and voriconazole). Grapefruit juice may also increase plasma concentrations of sirolimus (a major metabolite of temsirolimus) and should be avoided. If patients must be co-administered a strong CYP3A4 inhibitor, based on pharmacokinetic studies, a TORISEL dose reduction to 12.5 mg/week should be considered. This dose of TORISEL is predicted to adjust the AUC to the range observed without inhibitors. However, there are no clinical data with this dose adjustment in patients receiving strong CYP3A4 inhibitors. If the strong inhibitor is discontinued, a washout period of approximately 1 week should be allowed before the TORISEL dose is adjusted back to the dose used prior to initiation of the strong CYP3A4 inhibitor [see Warnings and Precautions (5.11) and Drug Interactions (7.2)].
Concomitant Strong CYP3A4 Inducers: The use of concomitant strong CYP3A4 inducers should be avoided (e.g. dexamethasone, phenytoin, carbamazepine, rifampin, rifabutin, rifampacin, phenobarbital). If patients must be co-administered a strong CYP3A4 inducer, based on pharmacokinetic studies, a TORISEL dose increase from 25 mg/week up to 50 mg/week should be considered. This dose of TORISEL is predicted to adjust the AUC to the range observed without inducers. However, there are no clinical data with this dose adjustment in patients receiving strong CYP3A4 inducers. If the strong inducer is discontinued the temsirolimus dose should be returned to the dose used prior to initiation of the strong CYP3A4 inducer [see Warnings and Precautions (5.11) and Drug Interactions (7.1)].
2.5 Instructions for PreparationTORISEL must be stored under refrigeration at 2°–8°C (36°–46°F) and protected from light. During handling and preparation of admixtures, TORISEL should be protected from excessive room light and sunlight. Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
In order to minimize the patient exposure to the plasticizer DEHP (di-2-ethylhexyl phthalate), which may be leached from PVC infusion bags or sets, the final TORISEL dilution for infusion should be stored in bottles (glass, polypropylene) or plastic bags (polypropylene, polyolefin) and administered through polyethylene-lined administration sets.
TORISEL 25 mg/mL injection must be diluted with the supplied diluent before further dilution in 0.9% Sodium Chloride Injection, USP.
Please note that both the TORISEL injection and diluent vials contain an overfill to ensure the recommended volume can be withdrawn.
Follow this two-step dilution process in an aseptic manner.
Step 1:
DILUTION OF TORISEL INJECTION 25 MG/ML WITH SUPPLIED DILUENT
Each Vial of Torisel (temsirolimus) must first be mixed with 1.8 mL of the enclosed diluent. The resultant solution contains 30 mg/3 mL (10 mg/mL). Mix well by inversion of the vial. Allow sufficient time for the air bubbles to subside. The solution should be clear to slightly turbid, colorless to light-yellow solution, essentially free from visual particulates.The concentrate-diluent mixture is stable below 25ºC for up to 24 hours.
Step 2:
DILUTION OF CONCENTRATE-DILUENT MIXTURE WITH 0.9% SODIUM CHLORIDE INJECTION, USP
Withdraw precisely the required amount of concentrate-diluent mixture containing temsirolimus 10 mg/mL as prepared in Step 1 from the vial (i.e., 2.5 mL for a temsirolimus dose of 25 mg) and further dilute into an infusion bag containing 250 mL of 0.9% Sodium Chloride Injection, USP. Mix by inversion of the bag or bottle, avoiding excessive shaking, as this may cause foaming.The resulting solution should be inspected visually for particulate matter and discoloration prior to administration. The admixture of TORISEL in 0.9% Sodium Chloride Injection, USP should be protected from excessive room light and sunlight.
2.6 Administration Administration of the final diluted solution should be completed within six hours from the time that TORISEL is first added to 0.9% Solution Chloride Injection, USP. TORISEL is infused over a 30- to 60-minute period once weekly. The use of an infusion pump is the preferred method of administration to ensure accurate delivery of the product. Appropriate administration materials should be composed of glass, polyolefin, or polyethylene to avoid excessive loss of product and diethylhexylpthalate (DEHP) extraction. The administration materials should consist of non-DEHP, non-polyvinylchloride (PVC) tubing with appropriate filter. In the case when a PVC administration set has to be used, it should not contain DEHP. An in-line polyethersulfone filter with a pore size of not greater than 5 microns is recommended for administration to avoid the possibility of particles bigger than 5 microns being infused. If the administration set available does not have an in-line filter incorporated, a polyethersulfone filter should be added at the set (i.e., an end-filter) before the admixture reaches the vein of the patient. Different end-filters can be used, ranging in filter pore size from 0.2 microns up to 5 microns. The use of both an in-line and end-filter is not recommended. TORISEL, when diluted, contains polysorbate 80, which is known to increase the rate of DEHP extraction from PVC. This should be considered during the preparation and administration of TORISEL, including storage time elapsed when in direct contact with PVC following constitution.Compatibilities and Incompatibilities
Undiluted TORISEL injection should not be added directly to aqueous infusion solutions. Direct addition of TORISEL injection to aqueous solutions will result in precipitation of drug. Always combine TORISEL injection with DILUENT for TORISEL before adding to infusion solutions. It is recommended that TORISEL be administered in 0.9% Sodium Chloride Injection after combining with diluent. The stability of TORISEL in other infusion solutions has not been evaluated. Addition of other drugs or nutritional agents to admixtures of TORISEL in 0.9% Sodium Chloride Injection has not been evaluated and should be avoided. Temsirolimus is degraded by both acids and bases, and thus combinations of temsirolimus with agents capable of modifying solution pH should be avoided.
Login To Your Free Account