FDA records indicate that there are no current recalls for this drug.
Are you a medical professional?
Trending Topics
Trepoxicam-7.5 Recall
Get an alert when a recall is issued.
Questions & Answers
Side Effects & Adverse Reactions
There is currently no warning information available for this product. We apologize for any inconvenience.
Legal Issues
There is currently no legal information available for this drug.
FDA Safety Alerts
There are currently no FDA safety alerts available for this drug.
Manufacturer Warnings
There is currently no manufacturer warning information available for this drug.
FDA Labeling Changes
There are currently no FDA labeling changes available for this drug.
Uses
1 INDICATIONS AND USAGE
1.1 Osteoarthritis (OA) MOBIC is indicated for relief of the signs and symptoms of osteoarthritis [see Clinical Studies
(14.1)].
1.2 Rheumatoid Arthritis (RA) MOBIC is indicated for relief of the signs and symptoms of rheumatoid arthritis [see Clinical Studies
(14.1)].
1.3 Juvenile Rheumatoid Arthritis (JRA) Pauciarticular and Polyarticular Course MOBIC is indicated for relief of the signs and symptoms of pauciarticular or polyarticular course Juvenile Rheumatoid Arthritis in patients 2 years of age and older [see Clinical Studies
(14.2)].
INDICATIONS FOR USE
Trepadone is intended for the clinical dietary management of the metabolic processes of pain disorders and inflammatory conditions, particularly those associated with joint pain.
CLINICAL EXPERIENCE
Administration of Trepadone has demonstrated significant reduction in symptoms of pain and inflammation in patients with acute and chronic pain when used for the dietary management of the metabolic processes associated with pain disorders and inflammatory conditions. Administration of Trepadone results in the induction and maintenance of pain relief in patients with pain disorders and inflammatory conditions associated with joint pain.
History
There is currently no drug history available for this drug.
Other Information
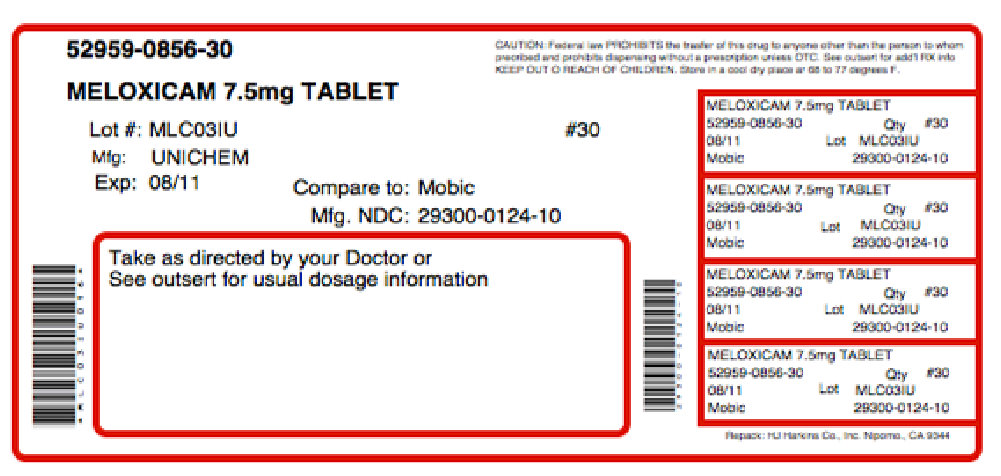
11 DESCRIPTION Meloxicam, an oxicam derivative, is a member of the enolic acid group of nonsteroidal anti-inflammatory drugs (NSAIDs). Each pastel yellow MOBIC tablet contains 7.5 mg or 15 mg meloxicam for oral administration. Each bottle of MOBIC oral suspension contains 7.5 mg meloxicam per 5 mL. Meloxicam is chemically designated as 4-hydroxy-2-methyl-N-(5-methyl-2-thiazolyl)-2H-1,2- benzothiazine-3-carboxamide-1,1-dioxide. The molecular weight is 351.4. Its empirical formula is C14H13N3O4S2 and it has the following structural formula:

Meloxicam is a pastel yellow solid, practically insoluble in water, with higher solubility observed in strong acids and bases. It is very slightly soluble in methanol. Meloxicam has an apparent partition coefficient (log P)app = 0.1 in n-octanol/buffer pH 7.4. Meloxicam has pKa values of 1.1 and 4.2. MOBIC is available as a tablet for oral administration containing 7.5 mg or 15 mg meloxicam, and as an oral suspension containing 7.5 mg meloxicam per 5 mL. The inactive ingredients in MOBIC tablets include colloidal silicon dioxide, crospovidone, lactose monohydrate, magnesium stearate, microcrystalline cellulose, povidone, and sodium citrate dihydrate. The inactive ingredients in MOBIC oral suspension include colloidal silicon dioxide, hydroxyethylcellulose, sorbitol, glycerol, xylitol, monobasic sodium phosphate (dihydrate), saccharin sodium, sodium benzoate, citric acid (monohydrate), raspberry flavor, and purified water.
PRODUCT DESCRIPTION
Primary Ingredients
Trepadone consists of a proprietary blend of amino acids, prostaglandin precursors in the form of omega-3 free fatty acids, glucosamine, chondroitin sulfate, cocoa, caffeine, cinnamon, and flavonoids in specific proportions. These ingredients fall into the category of Generally Regarded as Safe” (GRAS) as defined by the Food and Drug Administration (FDA) (Sections 201(s) and 409 of the Federal Food, Drug, and Cosmetic Act). A GRAS substance is distinguished from a food additive on the basis of the common knowledge about the safety of the substance for its intended use. The standard for an ingredient to achieve GRAS status requires not only technical demonstration of non-toxicity and safety, but also general recognition of safety through widespread usage and agreement of that safety by experts in the field. Many ingredients have been determined by the U.S. Food and Drug Administration (FDA) to be GRAS, and are listed as such by regulation, in Volume 21 Code of Federal Regulations (CFR) Sections 182, 184, and 186.
Amino Acids
Amino Acids are the building blocks of protein. All amino acids are GRAS listed as they have been ingested by humans for many thousands of years. The doses of the amino acids in Trepadone are equivalent to those found in the usual human diet. Patients with pain disorders may require an increased amount of certain amino acids that cannot be obtained from normal diet alone. Tryptophan, for example, is an obligatory amino acid. The body cannot make tryptophan and must obtain tryptophan from the diet. Tryptophan is needed to produce serotonin. Serotonin is required to reduce pain. Patients with pain disorders and inflammatory conditions have altered serotonin metabolism. Some patients with pain disorders and inflammatory conditions have a resistance to the use of tryptophan that is similar to the mechanism found in insulin resistance. Patients with pain disorders and inflammatory conditions cannot acquire sufficient tryptophan from the diet to alter the perception of pain and the inflammatory process without ingesting a prohibitively large amount of calories, particularly calories from protein.
Chondroitin Sulfate and Glucosamine
Chondroitin sulfate and glucosamine are the building blocks of joint cartilage and are GRAS listed as they have been ingested by humans for thousands of years. The doses of the chondroitin sulfate and glucosamine in Trepadone are equivalent to those found in the usual human diet. Patients with pain disorders, particularly of the joints, may require an increased amount of chondroitin sulfate and glucosamine that cannot be obtained from normal diet alone. Patients with pain disorders and inflammatory conditions, particularly of the joints have altered chondroitin sulfate and glucosamine metabolism. Some patients with pain disorders and inflammatory conditions of the joints have a resistance to the use of chondroitin sulfate and glucosamine. Patients with pain disorders and inflammatory conditions of the joints cannot acquire sufficient chondroitin sulfate and glucosamine from the diet to alter the pain and the inflammatory process of the joint without ingesting a prohibitively large amount of calories, particularly calories from protein.
Omega-3 Free Fatty Acids in the Form of Fish Oil
Omega-3 Free Fatty Acids in the Form of Fish Oil are the building blocks of prostaglandin precursors that control the inflammatory process. Omega-3 Free Fatty Acids in the Form of Fish Oil are GRAS listed as they have been ingested by humans for thousands of years. The doses of the Omega-3 Free Fatty Acids in the Form of Fish Oil in Trepadone are equivalent to those found in the usual human diet. Patients with pain disorders, particularly of the joints, may require an increased amount of Omega-3 Free Fatty Acids in the Form of Fish Oil that cannot be obtained from normal diet alone. Patients with pain disorders and inflammatory conditions, particularly of the joints have altered prostaglandin metabolism. Some patients with pain disorders and inflammatory conditions exhibit a resistance to the use of Omega-3 Free Fatty Acids in the Form of Fish Oil. Patients with pain disorders and inflammatory conditions of the joints cannot acquire sufficient Omega-3 Free Fatty Acids in the Form of Fish Oil from the diet to alter the pain and the inflammatory process of the joint without ingesting a prohibitively large amount of calories.
Flavonoids
Flavonoids are a group of phytochemical compounds found in all vascular plants including fruits and vegetables. They are a part of a larger class of compounds known as polyphenols. Many of the therapeutic or health benefits of colored fruits and vegetables, cocoa, red wine, and green tea are directly related to their flavonoid content. The specially formulated flavonoids found in Trepadone cannot be obtained from conventional foods in the necessary proportions to elicit a therapeutic response.
Other Ingredients
Trepadone contains the following inactive or other ingredients, as fillers, excipients, and colorings: magnesium stearate, microcrystalline cellulose, Maltodextrin NF, gelatin (as the capsule material).
Physical Description
Trepadone is a yellow to light brown powder. Trepadone contains L-Glutamine, L-Arginine, L-Histidine, and L-Serine, 5-Hydroxytryptophan as Griffonia Seed Extract, GABA, Choline Bitartrate, Cinnamon, Cocoa, Hydrolyzed Whey Protein, and Grape Seed Extract.
Sources
Trepoxicam-7.5 Manufacturers
-
Physician Therapeutics Llc
![Trepoxicam-7.5 (Meloxicam, Histidine) Kit [Physician Therapeutics Llc]](/wp-content/themes/bootstrap/assets/img/loading2.gif)
Trepoxicam-7.5 | Physician Therapeutics Llc
![Trepoxicam-7.5 (Meloxicam, Histidine) Kit [Physician Therapeutics Llc] Trepoxicam-7.5 (Meloxicam, Histidine) Kit [Physician Therapeutics Llc]](/wp-content/themes/bootstrap/assets/img/loading2.gif)
2 DOSAGE AND ADMINISTRATION
2.1 General Instructions Carefully consider the potential benefits and risks of MOBIC and other treatment options before deciding to use MOBIC. Use the lowest effective dose for the shortest duration consistent with individual patient treatment goals [see Warnings and Precautions (5.4)]. After observing the response to initial therapy with MOBIC, adjust the dose to suit an individual patient's needs. In adults, the maximum recommended daily oral dose of MOBIC is 15 mg regardless of formulation. In patients with hemodialysis, a maximum daily dosage of 7.5 mg is recommended [see Warnings and Precautions (5.6), Use in Specific Populations (8.7), and Clinical Pharmacology ( 12.3)]. MOBIC oral suspension 7.5 mg/5 mL or 15 mg/10 mL may be substituted for MOBIC tablets 7.5 mg or 15 mg, respectively. Shake the oral suspension gently before using. MOBIC may be taken without regard to timing of meals.
2.2 Osteoarthritis For the relief of the signs and symptoms of osteoarthritis the recommended starting and maintenance oral dose of MOBIC is 7.5 mg once daily. Some patients may receive additional benefit by increasing the dose to 15 mg once daily.
2.3 Rheumatoid Arthritis For the relief of the signs and symptoms of rheumatoid arthritis, the recommended starting and maintenance oral dose of MOBIC is 7.5 mg once daily. Some patients may receive additional benefit by increasing the dose to 15 mg once daily.
2.4 Juvenile Rheumatoid Arthritis (JRA) Pauciarticular and Polyarticular Course To improve dosing accuracy in smaller weight children, the use of the MOBIC oral suspension is recommended. MOBIC oral suspension is available in the strength of 7.5 mg/5 mL. For the treatment of juvenile rheumatoid arthritis, the recommended oral dose of MOBIC is 0.125 mg/kg once daily up to a maximum of 7.5 mg. There was no additional benefit demonstrated by increasing the dose above 0.125 mg/kg once daily in these clinical trials. Juvenile Rheumatoid Arthritis dosing using the oral suspension should be individualized based on the weight of the child:
0.125 mg/kg
Weight Dose (1.5 mg/mL) Delivered dose 12 kg (26 lb) 1.0 mL 1.5 mg 24 kg (54 lb) 2.0 mL 3.0 mg 36 kg (80 lb) 3.0 mL 4.5 mg 48 kg (106 lb 4.0 mL 6.0 mg ≥60 kg (132 lb) 5.0 mL 7.5 mgDOSAGE AND ADMINISTRATION
Recommended Administration
For the dietary management of the metabolic processes associated with pain disorders and inflammatory conditions, particularly joint pain. Take (2) capsules up to four times per day times daily or as directed by physician. As with most amino acid formulations Trepadone should be taken without food to increase the absorption of key ingredients.
Login To Your Free Account