Trizivir Recall
Get an alert when a recall is issued.
Questions & Answers
Side Effects & Adverse Reactions
There is currently no warning information available for this product. We apologize for any inconvenience.
Legal Issues
There is currently no legal information available for this drug.
FDA Safety Alerts
There are currently no FDA safety alerts available for this drug.
Manufacturer Warnings
There is currently no manufacturer warning information available for this drug.
FDA Labeling Changes
There are currently no FDA labeling changes available for this drug.
Uses
TRIZIVIR is indicated in combination with other antiretrovirals or alone for the treatment of HIV-1 infection.
Additional important information on the use of TRIZIVIR for treatment of HIV-1 infection:
-
•
-
TRIZIVIR is one of multiple products containing abacavir. Before starting TRIZIVIR, review medical history for prior exposure to any abacavir-containing product in order to avoid reintroduction in a patient with a history of hypersensitivity to abacavir
[see Warnings and Precautions (5.1), Adverse Reactions (6)].
-
•
-
TRIZIVIR is a fixed-dose combination of 3 nucleoside analogues: abacavir, lamivudine, and zidovudine and is intended only for patients whose regimen would otherwise include these 3 components.
-
•
-
Limited data exist on the use of TRIZIVIR alone in patients with higher baseline viral load levels (>100,000 copies/mL)
[see Clinical Studies (14)].
History
There is currently no drug history available for this drug.
Other Information
TRIZIVIR: TRIZIVIR Tablets contain the following 3 synthetic nucleoside analogues: abacavir sulfate (ZIAGEN), lamivudine (also known as EPIVIR or 3TC), and zidovudine (also known as RETROVIR, azidothymidine, or ZDV) with inhibitory activity against HIV-1.
TRIZIVIR Tablets are for oral administration. Each film-coated tablet contains the active ingredients 300 mg of abacavir as abacavir sulfate, 150 mg of lamivudine, and 300 mg of zidovudine, and the inactive ingredients magnesium stearate, microcrystalline cellulose, and sodium starch glycolate. The tablets are coated with a film (OPADRY® green 03B11434) that is made of FD&C Blue No. 2, hypromellose, polyethylene glycol, titanium dioxide, and yellow iron oxide.
Abacavir Sulfate: The chemical name of abacavir sulfate is (1S,cis)-4-[2-amino-6-(cyclopropylamino)-9H-purin-9-yl]-2-cyclopentene-1-methanol sulfate (salt) (2:1). Abacavir sulfate is the enantiomer with 1S, 4R absolute configuration on the cyclopentene ring. It has a molecular formula of (C14H18N6O)2•H2SO4 and a molecular weight of 670.76 daltons. It has the following structural formula:
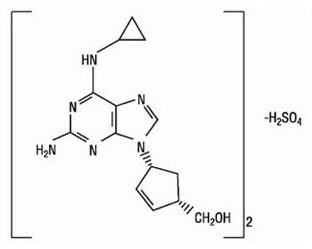
Abacavir sulfate is a white to off-white solid with a solubility of approximately 77 mg/mL in distilled water at 25°C.
In vivo, abacavir sulfate dissociates to its free base, abacavir. In this insert, all dosages for ZIAGEN (abacavir sulfate) are expressed in terms of abacavir.
Lamivudine: The chemical name of lamivudine is (2R,cis)-4-amino-1-(2-hydroxymethyl-1,3-oxathiolan-5-yl)-(1H)-pyrimidin-2-one. Lamivudine is the (-)enantiomer of a dideoxy analogue of cytidine. Lamivudine has also been referred to as (-)2′,3′-dideoxy, 3′-thiacytidine. It has a molecular formula of C8H11N3O3S and a molecular weight of 229.3 daltons. It has the following structural formula:
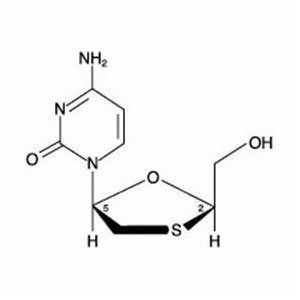
Lamivudine is a white to off-white crystalline solid with a solubility of approximately 70 mg/mL in water at 20°C.
Zidovudine: The chemical name of zidovudine is 3′-azido-3′-deoxythymidine. It has a molecular formula of C10H13N5O4 and a molecular weight of 267.24 daltons. It has the following structural formula:
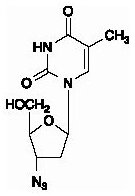
Zidovudine is a white to beige, crystalline solid with a solubility of 20.1 mg/mL in water at 25°C.
Sources