FDA records indicate that there are no current recalls for this drug.
Are you a medical professional?
Trending Topics
Vectibix Recall
Get an alert when a recall is issued.
Questions & Answers
Side Effects & Adverse Reactions
There is currently no warning information available for this product. We apologize for any inconvenience.
Legal Issues
There is currently no legal information available for this drug.
FDA Safety Alerts
There are currently no FDA safety alerts available for this drug.
Manufacturer Warnings
There is currently no manufacturer warning information available for this drug.
FDA Labeling Changes
There are currently no FDA labeling changes available for this drug.
Uses
Vectibix is indicated for the treatment of patients with wild-type KRAS (exon 2 in codons 12 or 13) metastatic colorectal cancer (mCRC) as determined by an FDA-approved test for this use:
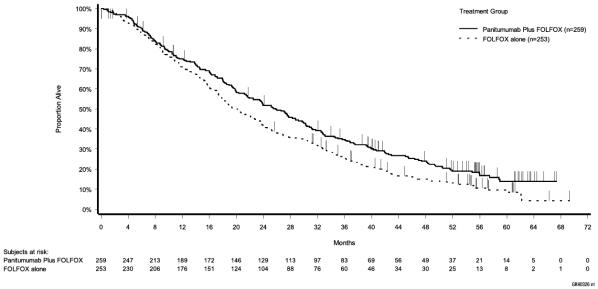
• As first-line therapy in combination with FOLFOX [see Clinical Studies (14.2)].
• As monotherapy following disease progression after prior treatment with fluoropyrimidine-, oxaliplatin-, and irinotecan-containing chemotherapy [see Clinical Studies (14.1)].
Limitation of Use: Vectibix is not indicated for the treatment of patients with RAS-mutant mCRC or for whom RAS mutation status is unknown [see Dosage and Administration (2.1), Warnings and Precautions (5.2), and Clinical Pharmacology (12.1)].
History
There is currently no drug history available for this drug.
Other Information
Vectibix (panitumumab) is a recombinant, human IgG2 kappa monoclonal antibody that binds specifically to the human epidermal growth factor receptor (EGFR). Panitumumab has an approximate molecular weight of 147 kDa. Panitumumab is produced in genetically engineered mammalian (Chinese hamster ovary) cells.
Vectibix is a sterile, colorless, pH 5.6 to 6.0 liquid for intravenous (IV) infusion, which may contain a small amount of visible translucent-to-white, amorphous, proteinaceous, panitumumab particulates. Each single-use 5 mL vial contains 100 mg of panitumumab, 29 mg sodium chloride, 34 mg sodium acetate, and Water for Injection, USP. Each single-use 10 mL vial contains 200 mg of panitumumab, 58 mg sodium chloride, 68 mg sodium acetate, and Water for Injection, USP. Each single-use 20 mL vial contains 400 mg of panitumumab, 117 mg sodium chloride, 136 mg sodium acetate, and Water for Injection, USP.
Sources
Vectibix Manufacturers
-
Amgen Inc
![Vectibix (Panitumumab) Solution [Amgen Inc]](/wp-content/themes/bootstrap/assets/img/loading2.gif)
Vectibix | Amgen Inc
![Vectibix (Panitumumab) Solution [Amgen Inc] Vectibix (Panitumumab) Solution [Amgen Inc]](/wp-content/themes/bootstrap/assets/img/loading2.gif)
2.1 Patient SelectionPrior to initiation of treatment with Vectibix, assess RAS mutational status in colorectal tumors and confirm the absence of a RAS mutation. Information on FDA-approved tests for the detection of KRAS mutations in patients with metastatic colorectal cancer is available at: http://www.fda.gov/CompanionDiagnostics.
2.2 Recommended DoseThe recommended dose of Vectibix is 6 mg/kg, administered as an intravenous infusion over 60 minutes, every 14 days. If the first infusion is tolerated, administer subsequent infusions over 30 to 60 minutes. Administer doses higher than 1000 mg over 90 minutes [see Dosage and Administration (2.4)].
Appropriate medical resources for the treatment of severe infusion reactions should be available during Vectibix infusions [see Warnings and Precautions (5.4)].
2.3 Dose ModificationsDose Modifications for Infusion Reactions [see Warnings and Precautions (5.4) and Adverse Reactions (6.1, 6.3)]
Reduce infusion rate by 50% in patients experiencing a mild or moderate (grade 1 or 2) infusion reaction for the duration of that infusion.
Terminate the infusion in patients experiencing severe infusion reactions. Depending on the severity and/or persistence of the reaction, permanently discontinue Vectibix.Dose Modifications for Dermatologic Toxicity [see Boxed Warning, Warnings and Precautions (5.1), and Adverse Reactions (6.1, 6.3)]
• Upon first occurrence of a grade 3 (NCI-CTC/CTCAE) dermatologic reaction, withhold 1 to 2 doses of Vectibix. If the reaction improves to < grade 3, reinitiate Vectibix at the original dose.
• Upon the second occurrence of a grade 3 (NCI-CTC/CTCAE) dermatologic reaction, withhold 1 to 2 doses of Vectibix. If the reaction improves to < grade 3, reinitiate Vectibix at 80% of the original dose.
• Upon the third occurrence of a grade 3 (NCI-CTC/CTCAE) dermatologic reaction, withhold 1 to 2 doses of Vectibix. If the reaction improves to < grade 3, reinitiate Vectibix at 60% of the original dose.
• Upon the fourth occurrence of a grade 3 (NCI-CTC/CTCAE) dermatologic reaction, permanently discontinue Vectibix.
Permanently discontinue Vectibix following the occurrence of a grade 4 dermatologic reaction or for a grade 3 (NCI-CTC/CTCAE) dermatologic reaction that does not recover after withholding 1 or 2 doses.
2.4 Preparation and AdministrationDo not administer Vectibix as an intravenous push or bolus.
Preparation
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration. Although Vectibix should be colorless, the solution may contain a small amount of visible translucent-to-white, amorphous, proteinaceous, panitumumab particulates (which will be removed by filtration; see below). Do not shake. Do not administer Vectibix if discoloration is observed.
Prepare the solution for infusion, using aseptic technique, as follows:
Withdraw the necessary amount of Vectibix for a dose of 6 mg/kg.
Dilute to a total volume of 100 mL with 0.9% sodium chloride injection, USP. Doses higher than 1000 mg should be diluted to 150 mL with 0.9% sodium chloride injection, USP. Do not exceed a final concentration of 10 mg/mL.
Mix diluted solution by gentle inversion. Do not shake.Administration
• Administer using a low-protein-binding 0.2 μm or 0.22 μm in-line filter.
• Vectibix must be administered via infusion pump.
◦ Flush line before and after Vectibix administration with 0.9% sodium chloride injection, USP, to avoid mixing with other drug products or intravenous solutions. Do not mix Vectibix with, or administer as an infusion with, other medicinal products. Do not add other medications to solutions containing panitumumab.◦ Infuse doses of 1000 mg or lower over 60 minutes through a peripheral intravenous line or indwelling intravenous catheter. If the first infusion is tolerated, administer subsequent infusions over 30 to 60 minutes. Administer doses higher than 1000 mg over 90 minutes.
• Use the diluted infusion solution of Vectibix within 6 hours of preparation if stored at room temperature, or within 24 hours of dilution if stored at 2° to 8°C (36º to 46ºF). DO NOT FREEZE.
• Discard any unused portion remaining in the vial.
Login To Your Free Account