FDA records indicate that there are no current recalls for this drug.
Are you a medical professional?
Trending Topics
Vetprofen Recall
Get an alert when a recall is issued.
Questions & Answers
Side Effects & Adverse Reactions
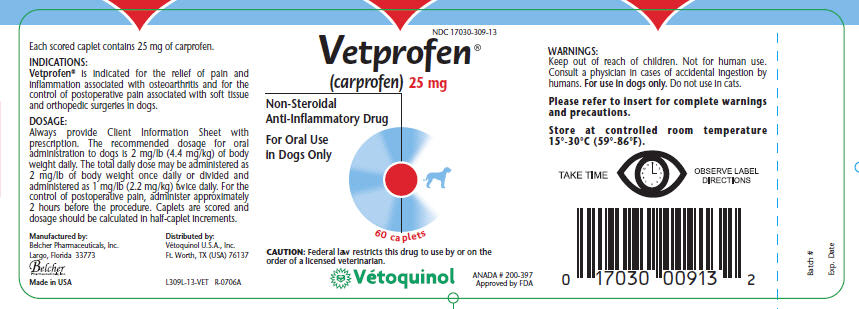
Keep out of reach of children. Not for human use. Consult a physician in cases of accidental ingestion by humans. For use in dogs only. Do not use in cats.
All dogs should undergo a thorough history and physical examination before initiation of NSAID therapy. Appropriate laboratory tests to establish hematological and serum biochemical baseline data prior to, and periodically during, administration of any NSAID should be considered. Owners should be advised to observe for signs of potential drug toxicity (see Information for Dog Owners, Adverse Reactions, Animal Safety and Post-Approval Experience).
Legal Issues
There is currently no legal information available for this drug.
FDA Safety Alerts
There are currently no FDA safety alerts available for this drug.
Manufacturer Warnings
There is currently no manufacturer warning information available for this drug.
FDA Labeling Changes
There are currently no FDA labeling changes available for this drug.
Uses
Vetprofen is indicated for the relief of pain and inflammation associated with osteoarthritis and for the control of postoperative pain associated with soft tissue and orthopedic surgeries in dogs.
History
There is currently no drug history available for this drug.
Other Information
Vetprofen (carprofen) is a non-steroidal anti-inflammatory drug (NSAID) of the propionic acid class that includes ibuprofen, naproxen, and ketoprofen. Carprofen is the nonproprietary designation for a substituted carbazole, 6-chloro-α-methyl-9H-carbazole-2-acetic acid. The empirical formula is C15H12ClNO2 and the molecular weight 273.72. The chemical structure of carprofen is:

Carprofen is a white, crystalline compound. It is freely soluble in ethanol, but practically insoluble in water at 25°C.
Sources
Vetprofen Manufacturers
-
Vetoquinol Usa Inc
![Vetprofen (Carprofen) Tablet [Vetoquinol Usa Inc]](/wp-content/themes/bootstrap/assets/img/loading2.gif)
Vetprofen | Vetoquinol Usa Inc
![Vetprofen (Carprofen) Tablet [Vetoquinol Usa Inc] Vetprofen (Carprofen) Tablet [Vetoquinol Usa Inc]](/wp-content/themes/bootstrap/assets/img/loading2.gif)
Always provide Client Information Sheet with prescription. The recommended dosage for oral administration to dogs is 2 mg/lb (4.4 mg/kg) of body weight daily. The total daily dose may be administered as 2 mg/lb of body weight once daily or divided and administered as 1 mg/lb (2.2 mg/kg) twice daily. For the control of postoperative pain, administer approximately 2 hours before the procedure. Caplets are scored and dosage should be calculated in half-caplet increments.
Login To Your Free Account