FDA records indicate that there are no current recalls for this drug.
Are you a medical professional?
Trending Topics
Xenical Recall
Get an alert when a recall is issued.
Questions & Answers
Side Effects & Adverse Reactions
There is currently no warning information available for this product. We apologize for any inconvenience.
Legal Issues
There is currently no legal information available for this drug.
FDA Safety Alerts
There are currently no FDA safety alerts available for this drug.
Manufacturer Warnings
There is currently no manufacturer warning information available for this drug.
FDA Labeling Changes
There are currently no FDA labeling changes available for this drug.
Uses
XENICAL is indicated for obesity management including weight loss and weight maintenance when used in conjunction with a reduced-calorie diet. XENICAL is also indicated to reduce the risk for weight regain after prior weight loss. XENICAL is indicated for obese patients with an initial body mass index (BMI) ≥30 kg/m2 or ≥27 kg/m2 in the presence of other risk factors (e.g., hypertension, diabetes, dyslipidemia).
Table 1 illustrates body mass index (BMI) according to a variety of weights and heights. The BMI is calculated by dividing weight in kilograms by height in meters squared. For example, a person who weighs 180 lbs and is 5'5" would have a BMI of 30.
|
 |
History
There is currently no drug history available for this drug.
Other Information
XENICAL (orlistat) is a gastrointestinal lipase inhibitor for obesity management that acts by inhibiting the absorption of dietary fats.
Orlistat is (S)-2-formylamino-4-methyl-pentanoic acid (S)-1-[[(2S, 3S)-3-hexyl-4-oxo-2-oxetanyl] methyl]-dodecyl ester. Its empirical formula is C29H53NO5, and its molecular weight is 495.7. It is a single diastereomeric molecule that contains four chiral centers, with a negative optical rotation in ethanol at 529 nm. The structure is:

Orlistat is a white to off-white crystalline powder. Orlistat is practically insoluble in water, freely soluble in chloroform, and very soluble in methanol and ethanol. Orlistat has no pKa within the physiological pH range.
XENICAL is available for oral administration as a turquoise hard-gelatin capsule. The capsule is imprinted with black. Each capsule contains a pellet formulation consisting of 120 mg of the active ingredient, orlistat, as well as the inactive ingredients microcrystalline cellulose, sodium starch glycolate, sodium lauryl sulfate, povidone, and talc. The capsule shell contains gelatin, titanium dioxide, and FD&C Blue No. 2 with black printing ink containing pharmaceutical grade shellac, dehydrated alcohol, isopropyl alcohol, butyl alcohol, propylene glycol, strong ammonium solution, potassium hydroxide and black iron oxide.
Sources
Xenical Manufacturers
-
Genentech, Inc.
![Xenical (Orlistat) Capsule [Genentech, Inc.]](/wp-content/themes/bootstrap/assets/img/loading2.gif)
Xenical | Csl Behring Gmbh
![Xenical (Orlistat) Capsule [Genentech, Inc.] Xenical (Orlistat) Capsule [Genentech, Inc.]](/wp-content/themes/bootstrap/assets/img/loading2.gif)
For Intravenous Use Only.
Administer Berinert at a dose of 20 International Units (IU) per kg body weight by intravenous injection. Doses lower than 20 IU/kg body weight should not be administered.
Berinert is provided as a freeze-dried powder for reconstitution with the Sterile Water for Injection, USP provided. Store the vial in the original carton in order to protect from light. Do not freeze.
2.1 Preparation and Handling Check the expiration date on the product vial label. Do not use beyond the expiration date. Prepare and administer using aseptic techniques [see Dosage and Administration (2.2)]. Use a silicone-free syringe for reconstitution and administration of Berinert. After reconstitution and prior to administration, inspect Berinert visually for particulate matter and discoloration. The reconstituted solution should be colorless, clear, and free from visible particles. Do not use if the solution is cloudy, discolored, or contains particulates. The Berinert vial is for single use only. Berinert contains no preservative. Any product that has been reconstituted should be used promptly. The reconstituted solution must be used within 8 hours. Discard partially used vials. Do not freeze the reconstituted solution. 2.2 Reconstitution and AdministrationEach Berinert vial containing 500 IU of C1 esterase inhibitor as a lyophilized concentrate for reconstitution with 10 mL of Sterile Water for Injection, USP provided.
Use either the Mix2Vial® transfer set provided with Berinert [see How Supplied/Storage and Handling (16.1)] or a commercially available double-ended needle and vented filter spike.
Reconstitution
The procedures below are provided as general guidelines for the reconstitution and administration of Berinert.
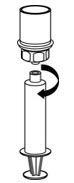
1. Ensure that the Berinert vial and diluent vial are at room temperature. 2. Place the Berinert vial, diluent vial and Mix2Vial transfer set on a flat surface. 3. Remove the flip caps from the Berinert and diluent vials. Wipe the vial stoppers with the alcohol swab provided. Allow to dry prior to opening the Mix2Vial transfer set package. 4. Open the Mix2Vial transfer set package by peeling away the lid (Figure 1). Leave the Mix2Vial transfer set in the clear package.
Figure 1 5. Place the diluent vial on a flat surface and hold the vial tightly. Grip the Mix2Vial transfer set together with the clear package and push the plastic spike at the blue end of the Mix2Vial transfer set firmly through the center of the stopper of the diluent vial (Figure 2).
Figure 2 6. Carefully remove the clear package from the Mix2Vial transfer set. Make sure that you pull up only the clear package, and not the Mix2Vial transfer set (Figure 3).
Figure 3 7. With the Berinert vial placed firmly on a flat surface, invert the diluent vial with the Mix2Vial transfer set attached and push the plastic spike of the transparent adapter firmly through the center of the stopper of the Berinert vial (Figure 4). The diluent will automatically transfer into the Berinert vial.
Figure 4 8. With the diluent and Berinert vial still attached to the Mix2Vial transfer set, gently swirl the Berinert vial to ensure that the Berinert is fully dissolved (Figure 5). Do not shake the vial.
Figure 5 9. With one hand, grasp the Berinert-side of the Mix2Vial transfer set and with the other hand grasp the blue diluent-side of the Mix2Vial transfer set and unscrew the set into two pieces (Figure 6).
Figure 6 10. Carefully look at reconstituted solution in each vial of Berinert. It should be colorless, clear, and free from visible particles. Do not use the vial if the liquid looks cloudy, contains particles, or has changed color. Do not use if the expiration date on the label has expired. 11. Draw air into an empty, sterile syringe. Use a silicone-free syringe. While the Berinert vial is upright, screw the syringe to the Mix2Vial transfer set. Inject air into the Berinert vial. While keeping the syringe plunger pressed, invert the system upside down and draw the concentrate into the syringe by pulling the plunger back slowly (Figure 7).
Figure 7 12. Now that the concentrate has been transferred into the syringe, firmly grasp the barrel of the syringe (keeping the plunger facing down) and unscrew the syringe from the Mix2Vial transfer set (Figure 8). Attach the syringe to a suitable intravenous administration set.
Figure 8 13. If patient requires more than one vial, pool the contents of multiple vials into one syringe. A new unused Mix2Vial transfer set should be used for each Berinert vial. 14. Do not refrigerate after reconstitution. When reconstitution is carried out using aseptic technique, administration may begin within 8 hours, provided the solution has been stored at up to 25°C (77°F). Do not refrigerate or freeze the reconstituted solution. Only store the reconstituted product in the vial.Administration
Do not mix Berinert with other medicinal products. Administer Berinert by a separate infusion line. Use aseptic technique when administering Berinert. Use a silicone-free syringe. Follow recommended venipuncture guidelines for initiating intravenous therapy. Administer Berinert by slow intravenous injection at a rate of approximately 4 mL per minute. Please refer to the illustration in step 6 of the self-administration section in the Patient Product Information (PPI) section. For self-administration, provide the patient with instructions and training for intravenous injection outside of a clinic setting so patients may self-administer Berinert upon recognition of symptoms of an HAE attack [see Patient Counseling Information (17)]. After administration, immediately discard any unused product and all used disposable supplies in accordance with local requirements. -
A-s Medication Solutions Llc
![Xenical (Orlistat) Capsule [A-s Medication Solutions Llc]](/wp-content/themes/bootstrap/assets/img/loading2.gif)
Xenical | A-s Medication Solutions Llc
![Xenical (Orlistat) Capsule [A-s Medication Solutions Llc] Xenical (Orlistat) Capsule [A-s Medication Solutions Llc]](/wp-content/themes/bootstrap/assets/img/loading2.gif)
2.1 Recommended DosingThe recommended dose of XENICAL is one 120-mg capsule three times a day with each main meal containing fat (during or up to 1 hour after the meal).
The patient should be on a nutritionally balanced, reduced-calorie diet that contains approximately 30% of calories from fat. The daily intake of fat, carbohydrate, and protein should be distributed over three main meals. If a meal is occasionally missed or contains no fat, the dose of XENICAL can be omitted.
Because XENICAL has been shown to reduce the absorption of some fat-soluble vitamins and beta-carotene, patients should be counseled to take a multivitamin containing fat-soluble vitamins to ensure adequate nutrition [see Warnings and Precautions (5.1)]. The vitamin supplement should be taken at least 2 hours before or after the administration of XENICAL, such as at bedtime.
For patients receiving both XENICAL and cyclosporine therapy, administer cyclosporine 3 hours after XENICAL.
For patients receiving both XENICAL and levothyroxine therapy, administer levothyroxine and XENICAL at least 4 hours apart. Patients treated concomitantly with XENICAL and levothyroxine should be monitored for changes in thyroid function.
Doses above 120 mg three times a day have not been shown to provide additional benefit.
Based on fecal fat measurements, the effect of XENICAL is seen as soon as 24 to 48 hours after dosing. Upon discontinuation of therapy, fecal fat content usually returns to pretreatment levels within 48 to 72 hours.
Login To Your Free Account


![Xenical (Orlistat) Capsule [A-s Medication Solutions Llc]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=a9c47f08-bd02-4731-aaf7-42173155b2d0&name=4742-0.jpg)