FDA records indicate that there are no current recalls for this drug.
Are you a medical professional?
Trending Topics
Xeomin Recall
Get an alert when a recall is issued.
Questions & Answers
Side Effects & Adverse Reactions
There is currently no warning information available for this product. We apologize for any inconvenience.
Legal Issues
There is currently no legal information available for this drug.
FDA Safety Alerts
There are currently no FDA safety alerts available for this drug.
Manufacturer Warnings
There is currently no manufacturer warning information available for this drug.
FDA Labeling Changes
There are currently no FDA labeling changes available for this drug.
Uses
XEOMIN (incobotulinumtoxinA) is indicated for the treatment of adults with cervical dystonia to decrease the severity of abnormal head position and neck pain in both botulinum toxin-naïve and previously treated patients.
XEOMIN (incobotulinumtoxinA) is indicated for the treatment of adults with blepharospasm who were previously treated with onabotulinumtoxinA (Botox).
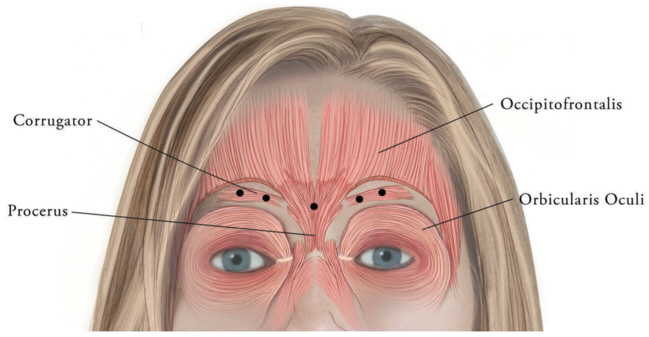
XEOMIN (incobotulinumtoxinA) is indicated for the temporary improvement in the appearance of moderate to severe glabellar lines associated with corrugator and/or procerus muscle activity in adult patients.
History
There is currently no drug history available for this drug.
Other Information
The active ingredient of XEOMIN is botulinum toxin type A produced from fermentation of Hall strain Clostridium botulinum serotype A. The botulinum toxin complex is purified from the culture supernatant and then the active ingredient is separated from the proteins (hemagglutinins and non-hemagglutinins) through a series of steps yielding the active neurotoxin with molecular weight of 150 kDa, without accessory proteins. XEOMIN is a sterile white to off-white lyophilized powder intended for intramuscular injection after reconstitution with preservative-free 0.9% Saline for Injection. One vial of XEOMIN contains 50 or 100 Units of incobotulinumtoxinA, 1 mg of human albumin, and 4.7 mg sucrose. One Unit corresponds to the mouse median lethal dose (LD50) when the reconstituted product is injected intraperitoneally into mice under defined conditions. The method for conducting the assay is specific to XEOMIN, Units of biological activity of XEOMIN cannot be converted into Units of any other botulinum toxin assessed with other specific assays.
Sources
Xeomin Manufacturers
-
Merz Pharmaceuticals, Llc
![Xeomin (Incobotulinumtoxina) Injection, Powder, Lyophilized, For Solution [Merz Pharmaceuticals, Llc]](/wp-content/themes/bootstrap/assets/img/loading2.gif)
Xeomin | Merz Pharmaceuticals, Llc
![Xeomin (Incobotulinumtoxina) Injection, Powder, Lyophilized, For Solution [Merz Pharmaceuticals, Llc] Xeomin (Incobotulinumtoxina) Injection, Powder, Lyophilized, For Solution [Merz Pharmaceuticals, Llc]](/wp-content/themes/bootstrap/assets/img/loading2.gif)
The potency Units of XEOMIN (incobotulinumtoxinA) for injection are specific to the preparation and assay method utilized. They are not interchangeable with other preparations of botulinum toxin products and, therefore, Units of biological activity of XEOMIN cannot be compared to or converted into Units of any other botulinum toxin products assessed with any other specific assay method [see Warnings and Precautions (5.2) and Description (11)].
2.1 Cervical DystoniaThe recommended initial total dose of XEOMIN for cervical dystonia is 120 Units. In a placebo-controlled trial utilizing initial XEOMIN doses of 120 Units and 240 Units, no meaningful difference in effectiveness was demonstrated between the doses [see Clinical Studies (14.1)]. In previously treated patients, their past dose, response to treatment, duration of effect, and adverse event history should be taken into consideration when determining the XEOMIN dose.
In the treatment of cervical dystonia, XEOMIN is usually injected into the sternocleidomastoid, levator scapulae, splenius capitis, scalenus, and/or the trapezius muscle(s). This list is not exhaustive, as any of the muscles responsible for controlling head position may require treatment [see Clinical Studies (14.1)]. The dose and number of injection sites in each treated muscle should be individualized based on the number and location of the muscle(s) to be treated, the degree of spasticity/dystonia, muscle mass, body weight, and response to any previous botulinum toxin injections.
The frequency of XEOMIN repeat treatments should be determined by clinical response, but should generally be no more frequent than every 12 weeks [see Clinical Studies (14.1)].
2.2 BlepharospasmThe recommended initial total dose of XEOMIN should be the same dose as the patient's previous treatment of onabotulinumtoxinA (Botox), although responses to XEOMIN and onabotulinumtoxinA (Botox) may differ in individual patients. In a placebo-controlled trial in which patients were dosed with the same number of Units as they had received previously with onabotulinumtoxinA (Botox), the mean dose per eye was about 33 Units (range 10-50 Units), and the mean number of injections per eye was 6. The maximum dose per eye in the controlled trials was 50 Units, with a range of 10-50 Units. In the controlled trial, few patients received a total dose of greater than 75 Units.
If the previous dose of Botox is not known, the initial dose of XEOMIN should be between 1.25-2.5 Units/injection site.
The total initial dose of XEOMIN in both eyes should not exceed 70 Units (35 Units/eye).
The number and location of injection sites should be based on the severity of blepharospasm, and previous dose and response to onabotulinumtoxinA (Botox) injections. Subsequent dosing should be tailored to the individual patient, based on response, up to a maximum dose of 35 Units per eye [see Clinical Studies 14.2]. XEOMIN dosing has not been established in patients with blepharospasm who have not been previously treated with onabotulinumtoxinA (Botox).
The frequency of XEOMIN repeat treatments should be determined by clinical response but should generally be no more frequent than every 12 weeks [see Clinical Studies (14.2)].
2.3 Glabellar LinesThe total recommended XEOMIN dose is 20 Units per treatment session divided into five equal intramuscular injections of 4 Units each. The five injection sites are: two injections in each corrugator muscle and one injection in the procerus muscle.
Retreatment with XEOMIN should be administered no more frequently than every three months.
Figure 1: Injection Sites for Glabellar Lines 2.4 Special PopulationsThe safety and effectiveness of XEOMIN in the treatment of cervical dystonia, blepharospasm, and glabellar lines in patients below 18 years of age have not been assessed [see Warnings and Precautions (5.1)].
2.5 Preparation and Reconstitution TechniquePrior to injection, reconstitute each vial of XEOMIN with sterile, preservative-free 0.9% Sodium Chloride Injection, USP. Draw up an appropriate amount of preservative-free 0.9% Sodium Chloride Injection, USP into a syringe (see Table 1). Clean the exposed portion of the rubber stopper of the vial with alcohol (70%) prior to insertion of the needle. Gently inject the saline solution into the vial. If the vacuum does not pull the solvent into the vial, then XEOMIN must be discarded. Gently mix XEOMIN with the saline by rotating the vial. Reconstituted XEOMIN is a clear, colorless solution free of particulate matter. XEOMIN should not be used if the reconstituted solution has a cloudy appearance or contains floccular or particulate matter.
Diluent volumes for reconstitution of XEOMIN are indicated in Table 1.
Table 1: Diluent Volumes for Reconstitution of XEOMIN Volume of Preservative-free 0.9% Sodium Chloride Injection, USP 50 Unit Vial:
Resulting dose in Units per 0.1 mL 100 Unit Vial:
Resulting dose in Units per 0.1 mL 0.25 mL 20 Units - 0.5 mL 10 Units 20 Units 1 mL 5 Units 10 Units 1.25 mL 4 Units 8 Units 2 mL 2.5 Units 5 Units 2.5 mL 2 Units 4 Units 4 mL 1.25 Units 2.5 Units 5 mL 1 Unit 2 Units 8 mL - 1.25 UnitsReconstituted XEOMIN solution should be administered within 24 hours after dilution. During this time period, reconstituted XEOMIN should be stored in a refrigerator 2-8°C (36-46°F) [see How Supplied/Storage and Handling (16.2)].
2.6 AdministrationReconstituted XEOMIN is intended for intramuscular injection only. After reconstitution, XEOMIN should be used for only one injection session and for only one patient.
If proposed injection sites are marked with a pen, the product must not be injected through the pen marks; otherwise a permanent tattooing effect may occur.
The number of injection sites is dependent upon the size of the muscle to be treated and the volume of reconstituted XEOMIN injected.
XEOMIN should be injected carefully when injected at sites close to sensitive structures, such as the carotid artery, lung apices and esophagus. Before administering XEOMIN, the physician should be familiar with the patient's anatomy and any anatomic alterations, e.g., due to prior surgical procedures.
Cervical Dystonia
A suitable sterile needle (e.g., 26-gauge (0.45 mm diameter), 37 mm length for superficial muscles; or 22-gauge (0.70 mm diameter), 75 mm length for injections into deeper muscles) should be used in the administration in the treatment of cervical dystonia.
Localization of the involved muscles with electromyographic guidance or nerve stimulation techniques may be useful.
Blepharospasm
A suitable sterile needle (e.g., 26-gauge (0.45 mm diameter), 37 mm length for superficial muscles; or 22-gauge (0.70 mm diameter), 75 mm length for injections into deeper muscles) should be used in the administration in the treatment of blepharospasm.
Glabellar Lines
A suitable sterile needle 30-33 gauge (0.3-0.2 mm diameter), 13 mm length should be used in the administration in the treatment of glabellar lines.
2.7 Monitoring to Assess EffectivenessThe median first onset of XEOMIN effect occurs within seven days after injection. The typical duration of effect of each treatment is up to 3 months; however, the effect may last significantly longer, or shorter, in individual patients.
-
Merz Aesthetics. Inc
![Xeomin (Incobotulinumtoxina) Injection, Powder, Lyophilized, For Solution [Merz Aesthetics. Inc]](/wp-content/themes/bootstrap/assets/img/loading2.gif)
Xeomin | Cardinal Health
![Xeomin (Incobotulinumtoxina) Injection, Powder, Lyophilized, For Solution [Merz Aesthetics. Inc] Xeomin (Incobotulinumtoxina) Injection, Powder, Lyophilized, For Solution [Merz Aesthetics. Inc]](/wp-content/themes/bootstrap/assets/img/loading2.gif)
• do not crush, chew, or break tablet • take with a full glass of water • this product can be administered without regard for the timing of meals • adults and children 12 years of age and over: 1 or 2 tablets every 12 hours. Do not exceed 4 tablets in 24 hours. • children under 12 years of age: do not use
Login To Your Free Account


![Xeomin (Incobotulinumtoxina) Injection, Powder, Lyophilized, For Solution [Merz Aesthetics. Inc]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=8ebc5b8c-a578-4f73-b5a1-3ea18a6b646e&name=image-01.jpg)