FDA records indicate that there are no current recalls for this drug.
Are you a medical professional?
Trending Topics
Dextran 75 Recall
Get an alert when a recall is issued.
Questions & Answers
Side Effects & Adverse Reactions
There is currently no warning information available for this product. We apologize for any inconvenience.
Legal Issues
There is currently no legal information available for this drug.
FDA Safety Alerts
There are currently no FDA safety alerts available for this drug.
Manufacturer Warnings
There is currently no manufacturer warning information available for this drug.
FDA Labeling Changes
There are currently no FDA labeling changes available for this drug.
Uses
Technetium Tc99m Dextran by intravenous administration is indicated as a cardiac blood pool imaging agent and as an adjunct in the diagnosis of pericardial effusion, ventricular aneurysm, or GI Bleed
History
There is currently no drug history available for this drug.
Other Information
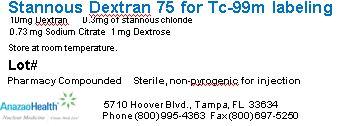
AnazaoHealth supplies compounded Dextran 75 for the preparation of Tc-99m Dextran 75. Each reaction vial contains 10 mg of Dextran 75, 0.30mg of stannous chloride, 0.73 mg Sodium Citrate and 1 mg of dextrose (lyophilized mixture, under nitrogen atmosphere), per unit dose vial.
Dextran, when labeled with technetium Tc99m and given intravenously, is distributed throughout the body in much the same way as the patient’s serum, and serves as a suitable tracer with which to transiently image the vascular compartment
Sources
Dextran 75 Manufacturers
-
Anazaohealth Corporation
![Dextran 75 Injection, Powder, Lyophilized, For Solution [Anazaohealth Corporation]](/wp-content/themes/bootstrap/assets/img/loading2.gif)
Dextran 75 | Anazaohealth Corporation
![Dextran 75 Injection, Powder, Lyophilized, For Solution [Anazaohealth Corporation] Dextran 75 Injection, Powder, Lyophilized, For Solution [Anazaohealth Corporation]](/wp-content/themes/bootstrap/assets/img/loading2.gif)
To prepare injection, up to 40 mCi of an oxidant-free sodium pertechnetate Tc 99m solution is aseptically injected into the vial, minimum volume 1ml, mix gently and let Dextran dissolve completely for 10 minutes
Storage and HandlingInjection should be administered within 6 hours after preparation. Before and after reconstitution- Store at room temperature
Login To Your Free Account